Final ID: Mo3074
AI-enhanced Electrocardiographic Evaluation of Left Ventricular Ejection Fraction and Outflow Tract Gradient in Hypertrophic Cardiomyopathy
Abstract Body (Do not enter title and authors here): Background: Longitudinal assessments of left ventricular ejection fraction (LVEF) and left ventricular outflow tract (LVOT) gradient have become critical in the management of HCM with the emergence of cardiac myosin inhibitors (CMI) but rely on repeated echocardiography. We developed AI-enhanced tools to evaluate these echocardiographic parameters using images/photos of 12-lead ECGs and 1-lead ECG signals captured by portable devices.
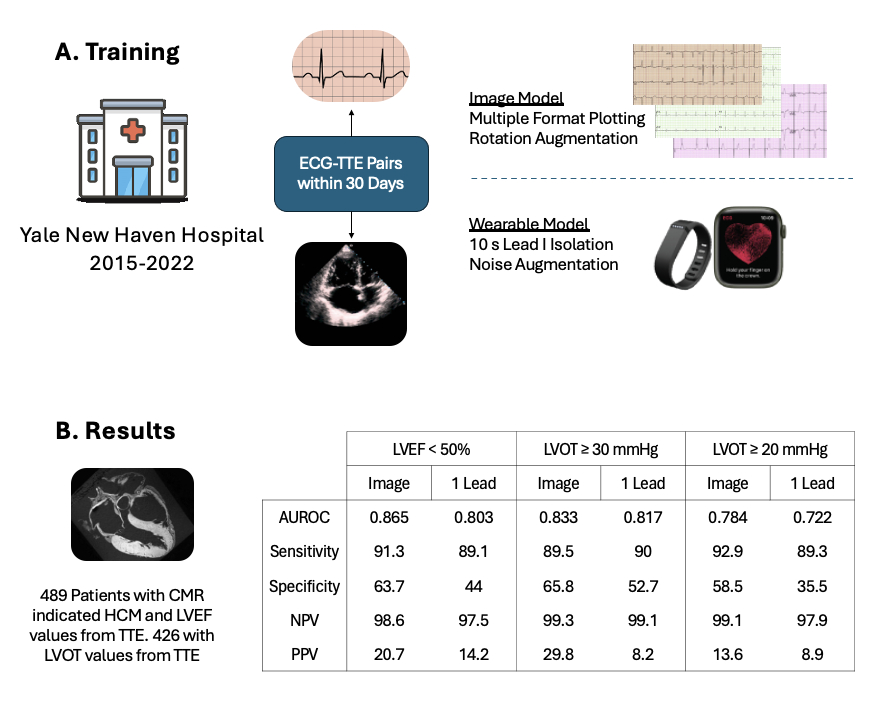
Methods: Using ECGs and paired echocardiograms recorded within 30 days at Yale during 2015-2022, we developed convolutional neural networks to predict continuous LVEF and LVOT gradients from 12-lead ECG images and lead I ECGs. We plotted 12-lead ECGs in varying layouts to ensure the image model was agnostic of real-world variations and augmented ECG signals with random Gaussian noises to improve the 1-lead model’s resilience to noisy acquisition. We leveraged resampling and weighted loss functions to optimize our regression models. We tested our models for detecting EF<50%, and LVOT≥30 and ≥20 mmHg – clinically important thresholds for treatment with CMI – on ECGs from a subset of patients with cardiac-MRI confirmed HCM.
Results: Models for LVEF and LVOT were developed in 98,778 and 11,135 unique patients, respectively. The LVEF models were tested on ECGs from 489 unique patients with HCM (age 61 [IQR:53-68] years, 182 [37.2%] women, 46 [9.4%] EF<50%) who were not in the development cohort. The image and 1-lead LVEF models had AUROCs of 0.865 and 0.803, sensitivities of 91.3% and 89.1%, and specificities of 63.7% and 44.0%, respectively. We tested the LVOT models in 426 patients with HCM (61[53-69] years, 163 [38.3%] women, 19 [4.5%] LVOT≥30, 28 [6.6%] LVOT≥20). The image and 1-lead LVOT models had AUROCs of 0.833 and 0.817, sensitivities of 89.5% and 90.0%, and specificities of 58.5% and 52.7%, respectively, for detecting LVOT≥30, and AUROCs of 0.784 and 0.722, sensitivities of 92.9% and 89.3%, and specificities of 58.5% and 35.5%, respectively for LVOT≥20.
Conclusion: AI applied to accessible modalities such as ECG images and 1-lead ECGs from portable devices may mitigate the burden of therapeutic monitoring for CMI in HCM, but further development and refinement are needed to fully validate their clinical utility as an efficient alternative to echocardiography.
Methods: Using ECGs and paired echocardiograms recorded within 30 days at Yale during 2015-2022, we developed convolutional neural networks to predict continuous LVEF and LVOT gradients from 12-lead ECG images and lead I ECGs. We plotted 12-lead ECGs in varying layouts to ensure the image model was agnostic of real-world variations and augmented ECG signals with random Gaussian noises to improve the 1-lead model’s resilience to noisy acquisition. We leveraged resampling and weighted loss functions to optimize our regression models. We tested our models for detecting EF<50%, and LVOT≥30 and ≥20 mmHg – clinically important thresholds for treatment with CMI – on ECGs from a subset of patients with cardiac-MRI confirmed HCM.
Results: Models for LVEF and LVOT were developed in 98,778 and 11,135 unique patients, respectively. The LVEF models were tested on ECGs from 489 unique patients with HCM (age 61 [IQR:53-68] years, 182 [37.2%] women, 46 [9.4%] EF<50%) who were not in the development cohort. The image and 1-lead LVEF models had AUROCs of 0.865 and 0.803, sensitivities of 91.3% and 89.1%, and specificities of 63.7% and 44.0%, respectively. We tested the LVOT models in 426 patients with HCM (61[53-69] years, 163 [38.3%] women, 19 [4.5%] LVOT≥30, 28 [6.6%] LVOT≥20). The image and 1-lead LVOT models had AUROCs of 0.833 and 0.817, sensitivities of 89.5% and 90.0%, and specificities of 58.5% and 52.7%, respectively, for detecting LVOT≥30, and AUROCs of 0.784 and 0.722, sensitivities of 92.9% and 89.3%, and specificities of 58.5% and 35.5%, respectively for LVOT≥20.
Conclusion: AI applied to accessible modalities such as ECG images and 1-lead ECGs from portable devices may mitigate the burden of therapeutic monitoring for CMI in HCM, but further development and refinement are needed to fully validate their clinical utility as an efficient alternative to echocardiography.
More abstracts on this topic:
12-lead electrocardiograms predict adverse cardiovascular outcomes of emergency department patients
Haimovich Julian, Kolossvary Marton, Alam Ridwan, Padros I Valls Raimon, Lu Michael, Aguirre Aaron
A Deep Learning Topic Analysis Approach for Enhancing Risk Assessment in Heart Failure Using Unstructured Clinical NotesAdejumo Philip, Pedroso Aline, Khera Rohan