Final ID: MP1034
Post-Discharge Outcomes and Healthcare Costs for Patients Hospitalized for Heart Failure with Transthyretin Amyloid Cardiomyopathy: Findings From GWTG–HF
Transthyretin amyloid cardiomyopathy (ATTR-CM) is associated with high risk for heart failure (HF) hospitalization. However, little is known regarding post-discharge outcomes and healthcare costs for patients with underlying ATTR-CM, as compared with the general HF population.
Methods:
We analyzed Medicare beneficiaries hospitalized for HF in the Get With The Guidelines-Heart Failure (GWTG-HF) registry and discharged alive from January 1, 2021, to June 30, 2023. Patients were compared according to the presence or absence of an ATTR-CM diagnosis, as documented in the GWTG-HF case report form. All-cause mortality, HF readmission, and all-cause readmission over 1-year post-discharge were assessed in unadjusted and adjusted risk models. Inpatient, outpatient (excluding medications), and total per-patient healthcare costs over the 1-year post-discharge were calculated from payments made by Medicare.
Results:
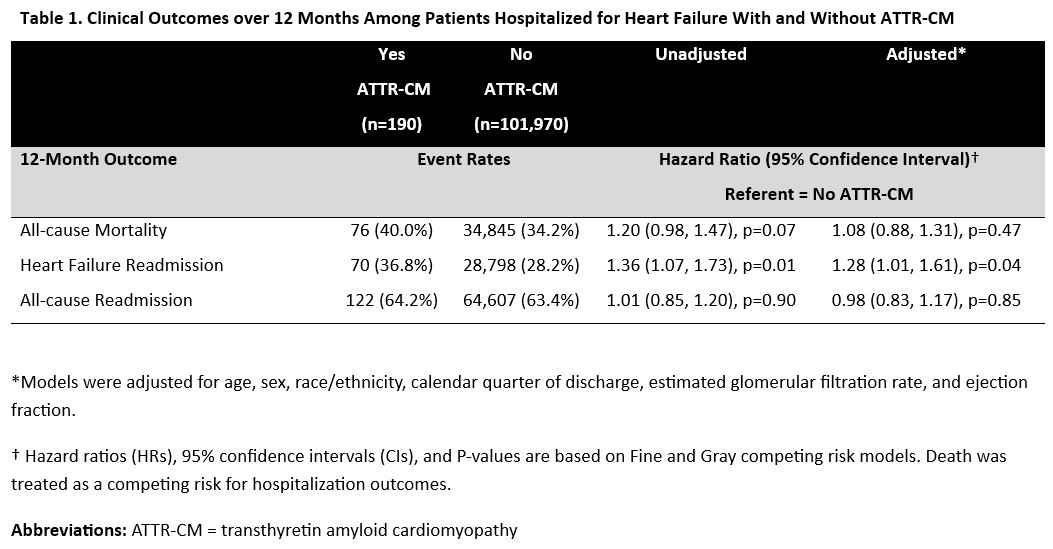
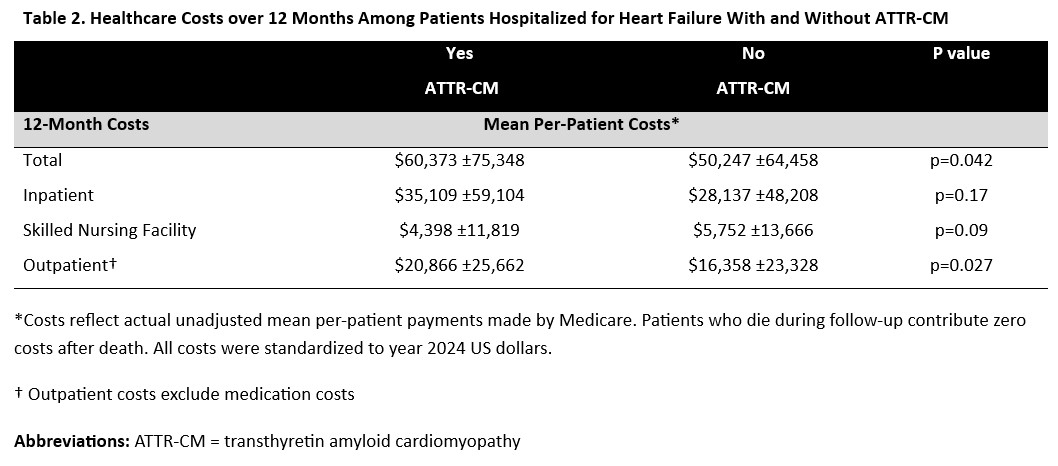
Among 102,160 patients across 563 US hospitals, 190 (0.2%) carried a diagnosis of ATTR-CM. Compared to those without ATTR-CM, patients with an ATTR-CM diagnosis were older (82 [76–87] vs 80 [73–87] years), more likely to be male (68.9% vs 47.0%), and had lower ejection fraction (45 [30–57] vs 53 [35–60]%). Patients with ATTR-CM were significantly more likely to be discharged on a mineralocorticoid receptor antagonist (29.5% vs 20.4%) and SGLT2 inhibitor (21.6% vs 14.1%), and significantly less likely to be discharged on a beta-blocker (52.1% vs 77.6%). After adjustment, ATTR-CM was associated with higher risk of 1-year HF readmission (36.8% vs. 28.2%; HR 1.28, 95% CI 1.01–1.61, p=0.04), but not all-cause mortality (40.0% vs. 34.2%; HR 1.08, 95% CI 0.88–1.31, p=0.47) or all-cause readmission (64.2% vs. 63.4%; HR 0.98, 95% CI 0.83–1.17, p=0.85) (Table 1). Mean 1-year total per-patient Medicare costs were higher among patients with ATTR-CM than those without ($60,373 vs $50,247; p=0.04), primarily driven by significant differences in outpatient costs ($20,866 vs $16,358]; p=0.03) (Table 2).
Conclusion:
Among older adults hospitalized for HF in the US, patients with underlying ATTR-CM experience similarly high rates of post-discharge mortality, but face greater risks of HF readmission and accrue higher post-discharge healthcare costs. These findings highlight a disproportionate clinical and economic burden of ATTR-CM compared with the general HF population, further supporting the need for earlier identification and tailored care strategies.
- Shoji, Satoshi ( Duke Clinical Research Institute , Chapel Hill , North Carolina , United States )
- Wright, Jason ( AstraZeneca , Bryn Mawr , Pennsylvania , United States )
- Vaduganathan, Muthiah ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Greene, Stephen ( Duke Clinical Research Institute , Durham , North Carolina , United States )
- Ikeaba, Uchechukwu ( Duke Clinical Research Institute , Durham , North Carolina , United States )
- Fonarow, Gregg ( UCLA MEDICAL CENTER , Los Angeles , California , United States )
- Selvaraj, Senthil ( Duke University , Durham , Pennsylvania , United States )
- Lewsey, Sabra ( Johns Hopkins University , Baltimore , Maryland , United States )
- Pandey, Ambarish ( UT Southwestern Medical Center , Dallas , Texas , United States )
- Khouri, Michel ( Duke University School of Medicine , Chapel Hill , North Carolina , United States )
- Alhanti, Brooke ( Duke University , Durham , North Carolina , United States )
- Mcdermott, Jim ( AstraZeneca / BioPharmaceuticals Medical CVRM , Wilmiton , Delaware , United States )
Meeting Info:
Session Info:
Cutting Edge Cardiomyopathies Clinical Research
Saturday, 11/08/2025 , 09:15AM - 10:30AM
Moderated Digital Poster Session
More abstracts on this topic:
Judge Daniel, Masri Ahmad, Obici Laura, Poulsen Steen, Sarswat Nitasha, Shah Keyur, Soman Prem, Cao Xiaofan, Wang Kevin, Pecoraro Maria, Tamby Jean-francois, Gillmore Julian, Katz Leonid, Fox Jonathan, Maurer Mathew, Alexander Kevin, Ambardekar Amrut, Cappelli Francesco, Fontana Marianna, Garcia-pavia Pablo, Grogan Martha, Hanna Mazen
A Case of Recurrent Acute Coronary Syndrome and Cardiogenic Shock due to Apolipoprotein A-IV AmyloidosisMuthukkumar Rashmi, Holmes Taylor, Friede Kevin
More abstracts from these authors:
Abdel Jawad Mohammad, Spertus John, Greene Stephen, Ikeaba Uchechukwu, Chiswell Karen, Fonarow Gregg, Chan Paul
Potential Patient Eligibility for Hospital at Home for Management of Worsening Heart Failure in the United StatesHaywood Hubert, Ayodele Iyanuoluwa, Fonarow Gregg, Alhanti Brooke, Van Spall Harriette, Pandey Ambarish, Lewsey Sabra, Butler Javed, Greene Stephen