Final ID: MP2076
Potential Patient Eligibility for Hospital at Home for Management of Worsening Heart Failure in the United States
Hospital at Home (HaH) is an emerging, patient-centered clinical model by which patients receive inpatient-level care at home. HaH may be particularly well-suited to the care of patients with worsening heart failure (WHF).
Research Questions
Our study sought to examine, if implemented widely, what proportion of US patients hospitalized with WHF would be eligible for HaH care. We also sought to determine the clinical and demographic differences between HaH eligible and ineligible populations.
Methods/Approach
Among US patients hospitalized for WHF in the Get With The Guidelines – Heart Failure (GWTG-HF) registry from 2021-2024, we applied generally accepted and/or required (by Medicare) social and clinical criteria for HaH to estimate the proportion of patients potentially eligible for HaH. We then further compared the demographics, vital signs and laboratory findings, comorbidities, mortality, and length of stay for the HaH eligible and ineligible groups.
Results/Data
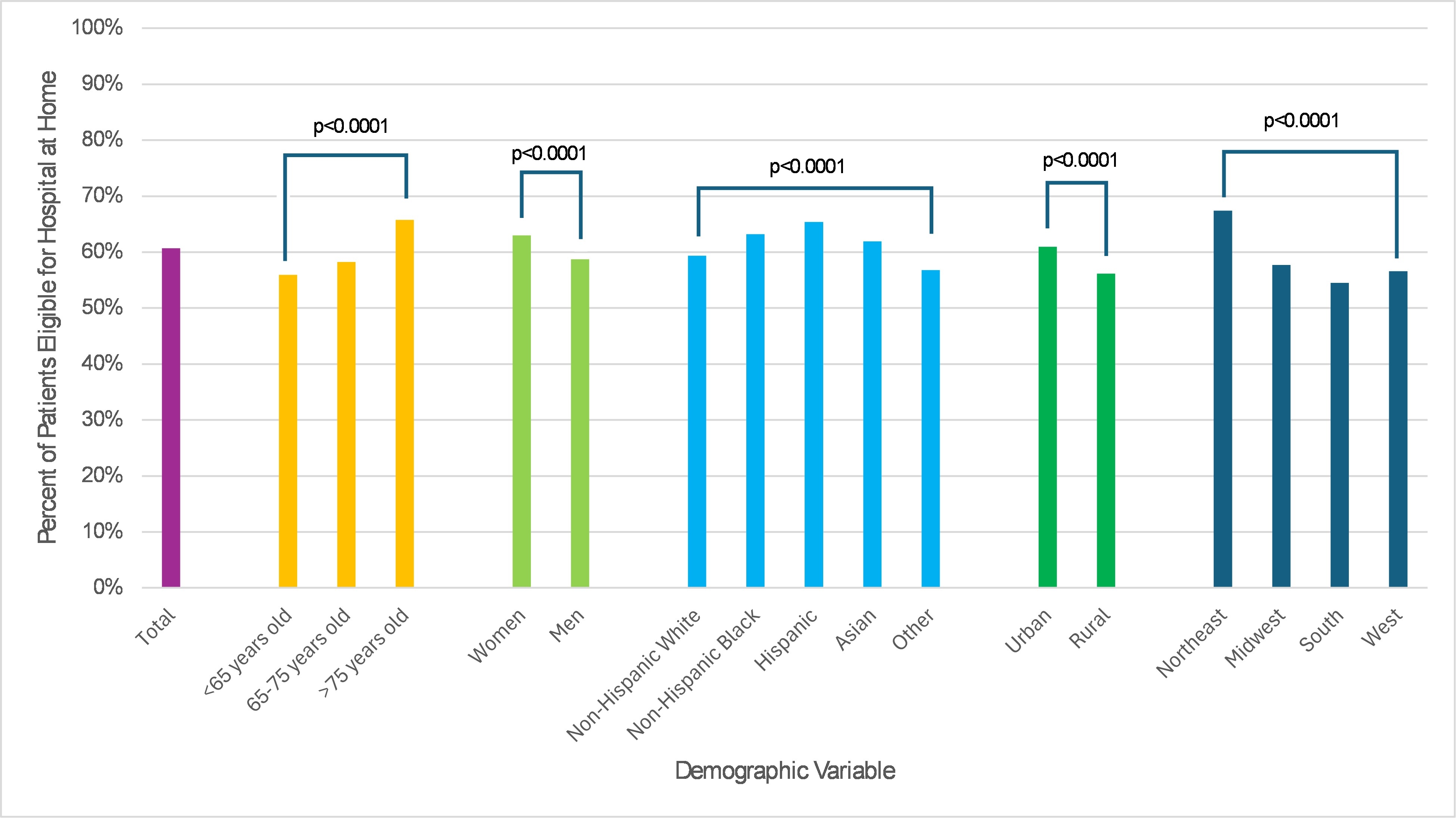
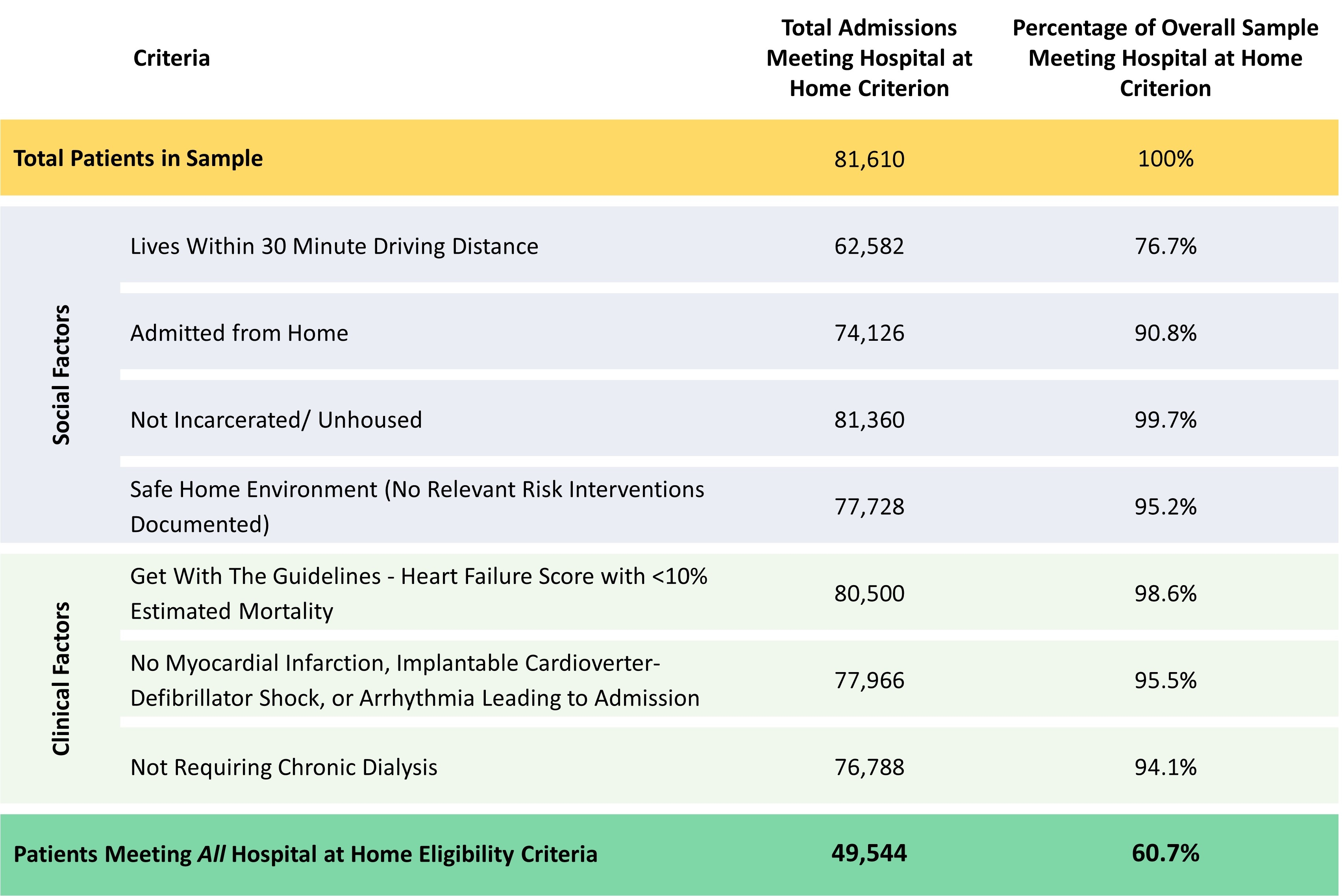
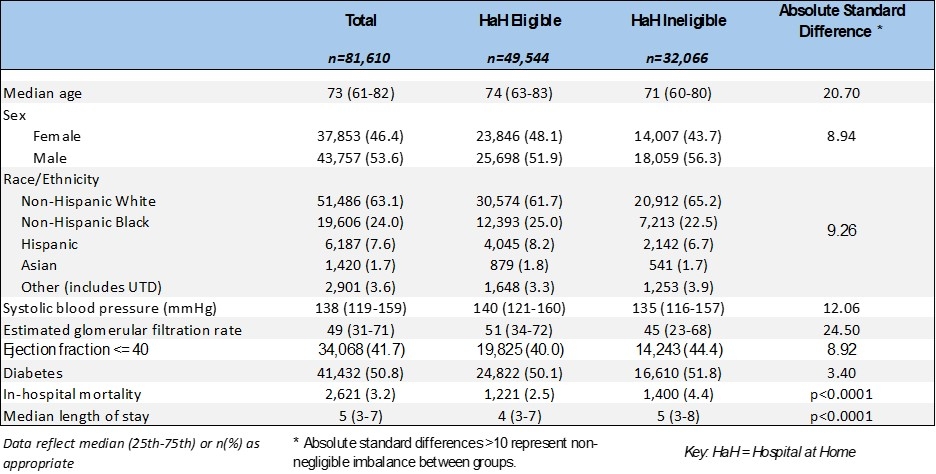
Among 81,610 patients hospitalized across 204 sites, 49,544 (60.7%) were projected as eligible for HaH (Table 1). Eligibility rates were >50% across demographic and geographic subgroups but tended to be higher among patients age >75 years, women, and Hispanic patients, as well as among patients hospitalized in urban areas and the Northeast US (Figure). Eligible patients were less likely to have a history of chronic kidney disease and had a lower median GWTG-HF risk score (Table 2). Patients eligible for HaH had lower in-hospital mortality and shorter length of stay (Table 2).
Conclusions
In this nationwide cohort of US patients hospitalized for WHF, approximately 6 out of 10 patients were projected as potentially eligible for HaH, with modest variability across demographic and geographic subgroups. Patients eligible for HaH demonstrated a lower risk clinical profile. HaH could conceivably be a viable treatment strategy for the majority of US patients with WHF, and national efforts to continue or expand HaH have the potential to substantially impact WHF care delivery.
- Haywood, Hubert ( Duke University Hospital , Durham , North Carolina , United States )
- Ayodele, Iyanuoluwa ( DCRI , Jonesboro , Georgia , United States )
- Fonarow, Gregg ( UCLA MEDICAL CENTER , Los Angeles , California , United States )
- Alhanti, Brooke ( Duke University , Durham , North Carolina , United States )
- Van Spall, Harriette ( McMaster University , Hamilton , Ontario , Canada )
- Pandey, Ambarish ( UT Southwestern Medical Center , Dallas , Texas , United States )
- Lewsey, Sabra ( Johns Hopkins University , Baltimore , Maryland , United States )
- Butler, Javed ( Baylor Scott and White Research , Dallas , Texas , United States )
- Greene, Stephen ( Duke Clinical Research Institute , Durham , North Carolina , United States )
Meeting Info:
Session Info:
From Molecules to Mindsets: Multidimensional Perspectives on Heart Failure
Monday, 11/10/2025 , 12:15PM - 01:00PM
Moderated Digital Poster Session
More abstracts on this topic:
Patel Prem, Milks Michael, Miller Andrew, Mehta Laxmi
Burden of Non-Rheumatic Valvular Heart Disease in High-income Asia Pacific from 1990-2019: A Benchmarking analysisShaikh Salomi, Amin Vishrant, Desai Hardik, Sharma Kamal, Shandilya Ashwinikumar, Patel Khushbu, Waqas Muhammad, Syed Saif, Lakkimsetti Mohit, Bhalodia Paritaben, Islam Hamza, Patel Juhi
More abstracts from these authors:
Shoji Satoshi, Wright Jason, Vaduganathan Muthiah, Greene Stephen, Ikeaba Uchechukwu, Fonarow Gregg, Selvaraj Senthil, Lewsey Sabra, Pandey Ambarish, Khouri Michel, Alhanti Brooke, Mcdermott Jim
Social Determinants of Health and Disparities in Guideline-Directed Medical Therapy Optimization for Heart FailureJacobs Joshua, Alhanti Brooke, Blanco Rosalia, Fonarow Gregg, Ayodele Iyanuoluwa, Bress Adam, Sterling Madeline, Pandey Ambarish, Derington Catherine, Zheutlin Alexander, Shah Kevin, Greene Stephen