Final ID: Mo1117
Early Adoption of Sodium-Glucose Cotransporter-2 Inhibitor Among Patients Hospitalized with Heart Failure and Preserved Ejection Fraction in the United States
Abstract Body (Do not enter title and authors here):
Introduction: In August 2021, the Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction (EMPEROR-Preserved) found that empagliflozin reduced rates of cardiovascular death and hospitalization by 21% in patients with heart failure with mildly reduced ejection fraction of 41-49% (HFmrEF) and with preserved ejection fraction of >50% (HFpEF). National trends in Sodium-Glucose Cotransporter-2 Inhibitors (SGLT2i) use among patients with HFmrEF and HFpEF following this trial are unknown.
Methods: We identified patients hospitalized for HFmrEF and HFpEF in the AHA Get With The Guidelines-Heart Failure registry between July 2021 and September 2023. We examined temporal trends in SGLT2i prescriptions at discharge after the publication of the EMPEROR-Preserved trial using the Cochran-Armitage trend test.
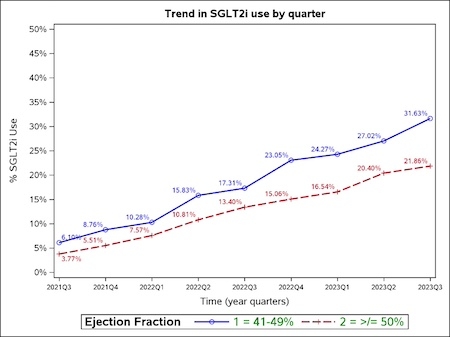
Results: Among 158,849 hospitalizations with an EF > 40% across 557 hospitals, 27,712 (17.4%) had HFmrEF, and 131,137 (82.6%) had HFpEF. Overall, 22,126 (13.9%) patients were discharged with a prescription for SGLT2i, with higher rates among those with HFmrEF (18.5% vs. 13.0% in those with HFpEF). Patients prescribed SGLT2i were more likely to be younger, male, of Black race, have type II diabetes, have Medicaid insurance, and be discharged from large hospitals. After publication of the EMPEROR-Preserved trial during Q3 2021, rates of SGLT2i prescription at hospital discharge increased from 4.2% in Q3 2021 to 23.5% in Q3 2023, with a 29% relative increase in the rate of SGLT2i prescription for each quarter (adjusted OR, 1.29 [95% CI: 1.28, 1.29]; P<.001). Prescription rates increased from 6.1% in Q3 2021 to 31.6% in Q3 2023 among patients with HFmrEF and from 3.8% to 21.9% for patients with HFpEF (Figure).
Conclusion: In the U.S., the use of SGLT2i at discharge has increased for patients hospitalized with HFmrEF and HFpEF following the publication of the EMPEROR-Preserved trial. However, the majority of eligible patients hospitalized for decompensated HF remain untreated with SGLT2i, indicating the need for further efforts to apply the scientific findings into clinical practice.
Introduction: In August 2021, the Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction (EMPEROR-Preserved) found that empagliflozin reduced rates of cardiovascular death and hospitalization by 21% in patients with heart failure with mildly reduced ejection fraction of 41-49% (HFmrEF) and with preserved ejection fraction of >50% (HFpEF). National trends in Sodium-Glucose Cotransporter-2 Inhibitors (SGLT2i) use among patients with HFmrEF and HFpEF following this trial are unknown.
Methods: We identified patients hospitalized for HFmrEF and HFpEF in the AHA Get With The Guidelines-Heart Failure registry between July 2021 and September 2023. We examined temporal trends in SGLT2i prescriptions at discharge after the publication of the EMPEROR-Preserved trial using the Cochran-Armitage trend test.
Results: Among 158,849 hospitalizations with an EF > 40% across 557 hospitals, 27,712 (17.4%) had HFmrEF, and 131,137 (82.6%) had HFpEF. Overall, 22,126 (13.9%) patients were discharged with a prescription for SGLT2i, with higher rates among those with HFmrEF (18.5% vs. 13.0% in those with HFpEF). Patients prescribed SGLT2i were more likely to be younger, male, of Black race, have type II diabetes, have Medicaid insurance, and be discharged from large hospitals. After publication of the EMPEROR-Preserved trial during Q3 2021, rates of SGLT2i prescription at hospital discharge increased from 4.2% in Q3 2021 to 23.5% in Q3 2023, with a 29% relative increase in the rate of SGLT2i prescription for each quarter (adjusted OR, 1.29 [95% CI: 1.28, 1.29]; P<.001). Prescription rates increased from 6.1% in Q3 2021 to 31.6% in Q3 2023 among patients with HFmrEF and from 3.8% to 21.9% for patients with HFpEF (Figure).
Conclusion: In the U.S., the use of SGLT2i at discharge has increased for patients hospitalized with HFmrEF and HFpEF following the publication of the EMPEROR-Preserved trial. However, the majority of eligible patients hospitalized for decompensated HF remain untreated with SGLT2i, indicating the need for further efforts to apply the scientific findings into clinical practice.
More abstracts on this topic:
Demographic Disparities in Tafamidis Treatment and Clinical Outcomes Across the United States
Cyrille-superville Nicole, Gaggin Hanna, Rosen Andrew, Udall Margarita, Hennum Liana, Gao Xingyu, Nagelhout Elizabeth, Keshishian Allison, Davis Margot
β1 Adrenergic Receptor Autoantibodies Promote Heart Failure Though Activation of Prostaglandin E2 Receptor EP1/Phosphodiesterase 4B PathwayCao Ning, Qiu Hui, Li Hongwei