Final ID: 4364317
Macrophage GPR68 Promotes Cell Infiltration and Collateral Arteriole Growth after Acute Myocardial Infarction
Abstract Body (Do not enter title and authors here): Background
GPR68, a mechanosensor and pH sensor, responds to shear stress and acidification and regulates mesenteric arteriole remodeling in response to pathological blood flow changes. Coronary collateral arteriole growth following myocardial infarction (MI) is critical for blood flow restoring to ischemic myocardium. The role of GPR68 in coronary collateral arteriole growth post-MI remains unknown.
Methods
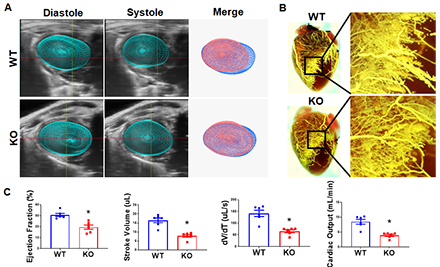
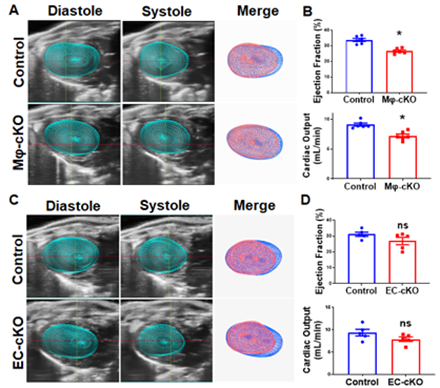
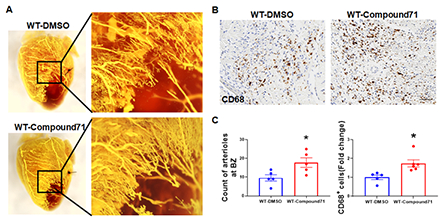
MI was induced in 12–16-week-old male C57BL/6J mice by permanent ligation of the left anterior descending artery. GPR68 expression was mapped using GPR68-tdTomato reporter mice at baseline and 3-d post-MI. Global and inducible Gpr68 knockout (KO) models (myeloid-specific, Mφ-cKO; endothelial-specific, EC-cKO) were used. Mice received tamoxifen chow (400 mg/kg) starting 14 days before MI until day 3 post-MI. Cardiac function was measured by 4D echocardiography. Coronary arteriole size and density were analyzed. GPR68 agonist Compound 71 (25 mg/kg/day) was given intraperitoneally for 3 days post-MI.
Results
GPR68-tdTomato reporter mice revealed significant accumulation of GPR68-positive macrophages in the border zone at 3-d post-MI. KO hearts showed decreased cardiac function at 3-d post-MI, with reduced macrophage infiltration, but no changes in cardiomyocyte size, capillary density, or infarct size, further confirming that macrophage Gpr68 plays a critical role in macrophage infiltration and cardiac function without affecting cell death. Strikingly, myocardial arterioles in the border zone significantly reduced in KO hearts 3-d post-MI. Transcriptome analysis showed mRNA expression of macrophage markers (CD68), chemokines (Ccr1/2/5, Ccl2/6/7/9), MMPs (Mmp8/12/13), and adhesion molecules (Itgb2, Itga4) was significantly lower in KO hearts at 3-d post-MI. Interestingly, Mφ-cKO recapitulated the global KO phenotype, but EC-cKO showed no difference, suggesting macrophage Gpr68 regulates macrophage recruitment and migration to the myocardium to promote coronary collateral arteriole growth post-MI. Consistently, GPR68 agonist Compound 71 treatment significantly improved cardiac function, macrophage infiltration, and arteriole density.
Conclusions
Macrophage GPR68 drives macrophage myocardial infiltration and collateral arteriole growth during acute MI. Pharmacologic activation with Compound 71 enhances macrophage infiltration, collateral arteriole growth, and cardiac functional recovery, underscoring GPR68’s therapeutic potential in ischemic heart disease.
GPR68, a mechanosensor and pH sensor, responds to shear stress and acidification and regulates mesenteric arteriole remodeling in response to pathological blood flow changes. Coronary collateral arteriole growth following myocardial infarction (MI) is critical for blood flow restoring to ischemic myocardium. The role of GPR68 in coronary collateral arteriole growth post-MI remains unknown.
Methods
MI was induced in 12–16-week-old male C57BL/6J mice by permanent ligation of the left anterior descending artery. GPR68 expression was mapped using GPR68-tdTomato reporter mice at baseline and 3-d post-MI. Global and inducible Gpr68 knockout (KO) models (myeloid-specific, Mφ-cKO; endothelial-specific, EC-cKO) were used. Mice received tamoxifen chow (400 mg/kg) starting 14 days before MI until day 3 post-MI. Cardiac function was measured by 4D echocardiography. Coronary arteriole size and density were analyzed. GPR68 agonist Compound 71 (25 mg/kg/day) was given intraperitoneally for 3 days post-MI.
Results
GPR68-tdTomato reporter mice revealed significant accumulation of GPR68-positive macrophages in the border zone at 3-d post-MI. KO hearts showed decreased cardiac function at 3-d post-MI, with reduced macrophage infiltration, but no changes in cardiomyocyte size, capillary density, or infarct size, further confirming that macrophage Gpr68 plays a critical role in macrophage infiltration and cardiac function without affecting cell death. Strikingly, myocardial arterioles in the border zone significantly reduced in KO hearts 3-d post-MI. Transcriptome analysis showed mRNA expression of macrophage markers (CD68), chemokines (Ccr1/2/5, Ccl2/6/7/9), MMPs (Mmp8/12/13), and adhesion molecules (Itgb2, Itga4) was significantly lower in KO hearts at 3-d post-MI. Interestingly, Mφ-cKO recapitulated the global KO phenotype, but EC-cKO showed no difference, suggesting macrophage Gpr68 regulates macrophage recruitment and migration to the myocardium to promote coronary collateral arteriole growth post-MI. Consistently, GPR68 agonist Compound 71 treatment significantly improved cardiac function, macrophage infiltration, and arteriole density.
Conclusions
Macrophage GPR68 drives macrophage myocardial infiltration and collateral arteriole growth during acute MI. Pharmacologic activation with Compound 71 enhances macrophage infiltration, collateral arteriole growth, and cardiac functional recovery, underscoring GPR68’s therapeutic potential in ischemic heart disease.
More abstracts on this topic:
A Perfect Storm: Simultaneous Pulmonary Embolism, STEMI, and Stroke via Paradoxical Embolism in a Hospitalized Patient on DVT Prophylaxis
Khan Abdul Allam, Thukral Jatin, Elgabry Ibrahim, Lamp Garron
A Novel Cardioprotective Mechanism in Myocardial Reperfusion Injury: Dual Neutrophil Modulation and ROS/HOCl Scavenging by an Atypical ChemokineZwissler Leon, Bernhagen Juergen, Cabrera-fuentes Hector Alejandro, Hernandez Resendiz Sauri, Yap En Ping, Schindler Lisa, Zhang Zhishen, Dickerhof Nina, Hampton Mark, Liehn Elisa, Hausenloy Derek