Final ID: MP977
Acoramidis Reduces All-Cause Mortality and Cardiovascular-Related Hospitalizations Through Month 42 in Transthyretin Amyloid Cardiomyopathy Across All Pre-specified Patient Subgroups
Acoramidis, an oral transthyretin (TTR) stabilizer achieving near-complete (≥90%) TTR stabilization, is now approved in the USA, EU, Japan, and UK for the treatment of TTR amyloid cardiomyopathy (ATTR-CM). In the 30-month phase 3 ATTRibute-CM study, acoramidis reduced all-cause mortality (ACM) or first cardiovascular-related hospitalization (CVH) vs placebo. Through Month 42 (M42) of the open-label extension (OLE; 30 months ATTRibute-CM + 12 months OLE), the clinical benefit of acoramidis was sustained, resulting in a 36% and 43% risk reduction in ACM and ACM/first CVH, respectively, compared with the placebo to acoramidis group
Research Question
Are the long-term clinical benefits of acoramidis on ACM and ACM/first CVH in ATTR-CM over 42 months consistent across patient subgroups?
Methods
ATTRibute-CM study design has been previously described. At the end of the 30-month double-blind period, patients enrolled into OLE continued acoramidis or switched from placebo to acoramidis; all patients received open-label acoramidis HCI 800 mg BID. Data cut was performed after 12 months in OLE. Subgroup analysis for ACM and ACM/first CVH through M42 were conducted for randomization stratification factors (genotype, NT-proBNP, and eGFR levels) and other pre-specified subgroups. ACM included death due to any cause, cardiac mechanical assist device placement, and heart transplant. CVH included CV hospitalizations (≥24 h) and urgent visits (<24 h) for decompensated heart failure requiring IV diuretics and was adjudicated by an independent Clinical Events Committee.
Results
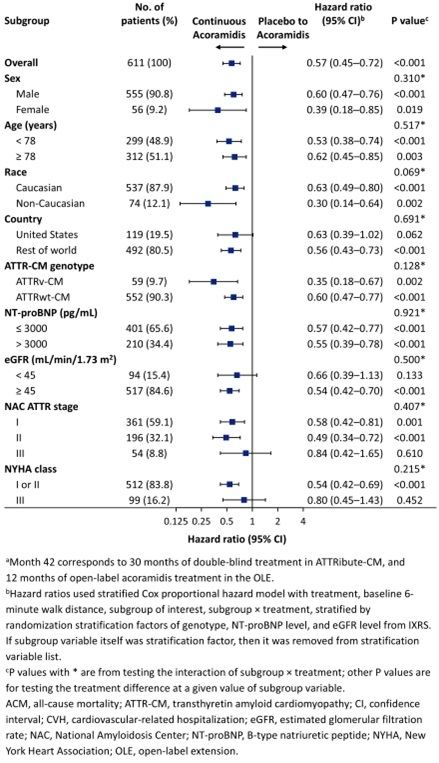
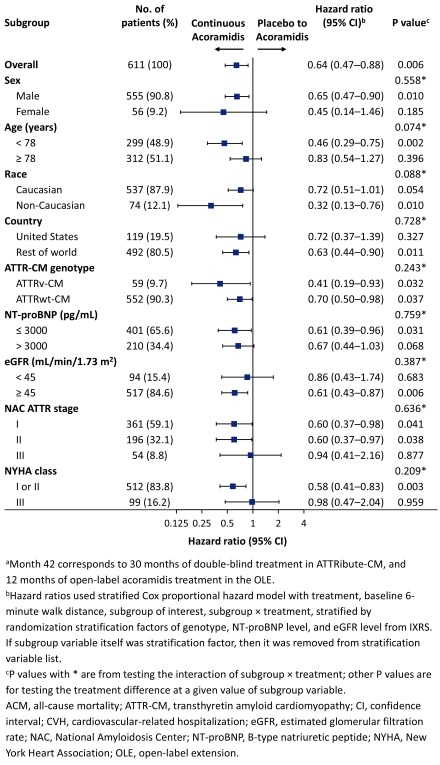
Overall, 389 patients enrolled in the OLE (263 continuous acoramidis and 126 placebo to acoramidis). The analyses of ACM/first CVH (Figure 1) and ACM (Figure 2) through M42 consistently favored patients initially randomized to acoramidis across all patient subgroups, including those based on sex, age, country, ATTR-CM genotype, baseline NT-proBNP, baseline eGFR, NYHA class, and NAC stage. The largest effect size for the reduction in ACM/first CVH (HR <0.50) was observed in the following subgroups: female, non-Caucasian patients, variant ATTR-CM, or NAC stage 2.
Conclusions
The long-term benefit of acoramidis was consistently shown in patients initially randomized to acoramidis treatment compared with placebo to acoramidis switch after 30 months across multiple clinically relevant subgroups, underscoring the importance of early initiation of acoramidis to reduce the long-term risk of ACM or CVH.
- Stern, Lily ( Cedars-Sinai Medical Center , Beverly Hills , California , United States )
- Fine, Nowell ( University of Calgary , Calgary , Alberta , Canada )
- Maurer, Mathew ( Columbia University , New York , New York , United States )
- Grogan, Martha ( Mayo Clinic , Rochester , Minnesota , United States )
- Ambardekar, Amrut ( University of Colorado , Aurora , Colorado , United States )
- Grodin, Justin ( University of Texas , Dallas , Texas , United States )
- Soman, Prem ( University of Pittsburgh , Pittsburgh , Pennsylvania , United States )
- Garcia-pavia, Pablo ( Hospital Universitario Puerta de Hierro Majadahonda , Madrid , Spain )
- Chen, Chris ( BridgeBio Pharma Inc. , San Francisco , California , United States )
- Siddhanti, Suresh ( BridgeBio Pharma Inc. , San Francisco , California , United States )
- Tamby, Jean-francois ( BridgeBio Pharma Inc. , San Francisco , California , United States )
- Fox, Jonathan ( BridgeBio Pharma Inc. , San Francisco , California , United States )
Meeting Info:
Session Info:
Heart Failure and Cardiomyopathy: From Bench to Bedside
Saturday, 11/08/2025 , 03:15PM - 04:25PM
Moderated Digital Poster Session
More abstracts on this topic:
Prendergast Heather, Khosla Shaveta, Kitsiou Spyros, Petzel Gimbar Renee, Freels Sally, Sanders Anissa, Daviglus Martha, Carter Barry, Del Rios Marina, Heinert Sara
A Polypill Strategy for Heart Failure with Reduced Ejection Fraction: The POLY-HF TrialPandey Ambarish, Wang Thomas, Keshvani Neil, Rizvi Syed Kazim, Jain Anand, Coellar Juan David, Drazner Mark, Gupta Deepak, Chandra Alvin, Zaha Vlad
More abstracts from these authors:
Davis Margot, Soman Prem, Kittleson Michelle, Berk John, Cao Xiaofan, Tamby Jean-francois, Castano Adam, Fox Jonathan, Shah Keyur, Grogan Martha, Griffin Jan, Sarswat Nitasha, Grodin Justin, Alexander Kevin, Judge Daniel, Gillmore Julian, Cappelli Francesco, Wright Richard
Acoramidis Reduces All-Cause Mortality (ACM) and Cardiovascular-Related Hospitalization (CVH): Initial Outcomes From the ATTRibute-CM Open-Label Extension (OLE) StudyJudge Daniel, Masri Ahmad, Obici Laura, Poulsen Steen, Sarswat Nitasha, Shah Keyur, Soman Prem, Cao Xiaofan, Wang Kevin, Pecoraro Maria, Tamby Jean-francois, Gillmore Julian, Katz Leonid, Fox Jonathan, Maurer Mathew, Alexander Kevin, Ambardekar Amrut, Cappelli Francesco, Fontana Marianna, Garcia-pavia Pablo, Grogan Martha, Hanna Mazen