Final ID: 4172052
Acoramidis Reduces All-Cause Mortality (ACM) and Cardiovascular-Related Hospitalization (CVH): Initial Outcomes From the ATTRibute-CM Open-Label Extension (OLE) Study
We report clinical outcomes following 36 and 42 months of treatment from the ongoing OLE.
Study Design and Methods: Participants who completed ATTRibute-CM were invited to enroll in the OLE. All OLE participants receive 800mg ACOR HCl BID.
Sample Size and Population Studied: Participants in the ATTRibute-CM OLE.
Intervention(s): 800 mg ACORAMIDIS (ACOR) HCl BID.
Power Calculations: Time-to-first event analyses using the Cox proportional hazards model were conducted at M36 and M42 for ACM, the composite of ACM or first CVH, and first CVH, and an Andersen-Gill (AG) analysis of instances of ACM and CVH as recurrent events was conducted at M36 and M42.
Primary Endpoint: Safety.
Secondary Endpoints: ACM, CVH.
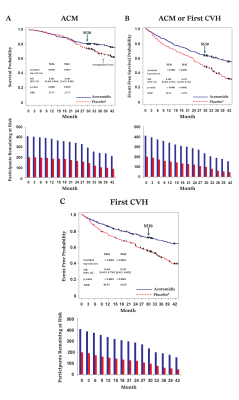
Outcomes: Compared to those previously treated with placebo (PBO), for those who received ACOR through M36 and M42, respectively, the risk of ACM was significantly reduced by 35.7% (hazard ratio [HR], 95% CI: 0.64, 0.46-0.64; p=0.009) and 33.7% (HR, 95% CI: 0.64, 0.47-0.88; p=0.006; Fig. 1A), composite ACM/first CVH by 34.3% (HR, 95% CI: 0.59, 0.46-0.75; p<0.0001) and 33.9% (HR, 95% CI: 0.57, 0.46-0.57; p<0.0001; Fig. 1B), and first CVH by 40.5% (HR, 95% CI: 0.56, 0.42-0.73; p<0.0001) and 41.0% (HR, 95% CI: 0.53, 0.41-0.69; p<0.0001; Fig. 1C). By an AG analysis at M36 and M42, respectively, ACM and recurrent CVH were reduced by 34% (HR, 95% CI: 0.66, 0.56-0.79) and 39% (HR, 95% CI: 0.61, 0.52, 0.72, both p<0.0001). Treatment effects of ACOR in the prior PBO arm demonstrate a trend of improved survival (Figure 1A, extrapolated vs observed curves from M30). The overall safety profile of ACOR remains consistent with the parent study. ACOR has statistically significant long-term benefits on ACM, ACM or first CVH, and first CVH at both M36 and M42 of the ongoing OLE, consistent with the ATTRibute-CM study.
- Judge, Daniel ( Medical University South Carolina , Charleston , South Carolina , United States )
- Masri, Ahmad ( Oregon Health & Science University , Portland , Oregon , United States )
- Obici, Laura ( Fondazione IRCCS Policlinico San Matteo , Pavia , Italy )
- Poulsen, Steen ( Aarhus University Hospital , Aarhus , Denmark )
- Sarswat, Nitasha ( University of Chicago Medicine , Chicago , Illinois , United States )
- Shah, Keyur ( Virginia Commonwealth University Health , Richmond , Virginia , United States )
- Soman, Prem ( University of Pittsburgh Medical Center , Pittsburgh , Pennsylvania , United States )
- Cao, Xiaofan ( BridgeBio Pharma, Inc. , San Francisco , California , United States )
- Wang, Kevin ( BridgeBio Pharma, Inc. , San Francisco , California , United States )
- Pecoraro, Maria ( BridgeBio Pharma, Inc. , San Francisco , California , United States )
- Tamby, Jean-francois ( BridgeBio Pharma, Inc. , San Francisco , California , United States )
- Gillmore, Julian ( University College London , London , United Kingdom )
- Katz, Leonid ( BridgeBio Pharma, Inc. , San Francisco , California , United States )
- Fox, Jonathan ( BridgeBio Pharma, Inc. , San Francisco , California , United States )
- Maurer, Mathew ( Columbia University Irving Medical Center , New York , New York , United States )
- Alexander, Kevin ( Stanford University School of Medicine , Palo Alto , California , United States )
- Ambardekar, Amrut ( University of Colorado , Aurora , Colorado , United States )
- Cappelli, Francesco ( Careggi University Hospital , Florence , Italy )
- Fontana, Marianna ( University College London , London , United Kingdom )
- Garcia-pavia, Pablo ( Hospital Universitario Puerta de Hierro Majadahonda , Madrid , Spain )
- Grogan, Martha ( Mayo Clinic , Rochester , Minnesota , United States )
- Hanna, Mazen ( Cleveland Clinic , Cleveland , Ohio , United States )
Meeting Info:
Session Info:
Monday, 11/18/2024 , 09:45AM - 11:00AM
Featured Science
More abstracts on this topic:
Jiang Meng, Guo Xinning
25-Year Decline in Aortic Aneurysm and Dissection Mortality in the U.S.: Impact of Endovascular Repair and Forecast to 2030Ali Manzer, Umar Haddaya, Nazir Tahira, Nizam Muhammad, Steafo Lark, Sharif Ayesha, Jehangir Hanzala, Arham Muhammad, Hamza Anfal, Hassan Arbaz, Amjad Ans, Ali Iman, Zuha Zuha
More abstracts from these authors:
Davis Margot, Soman Prem, Kittleson Michelle, Berk John, Cao Xiaofan, Tamby Jean-francois, Castano Adam, Fox Jonathan, Shah Keyur, Grogan Martha, Griffin Jan, Sarswat Nitasha, Grodin Justin, Alexander Kevin, Judge Daniel, Gillmore Julian, Cappelli Francesco, Wright Richard
Acoramidis Reduces All-Cause Mortality and Cardiovascular-Related Hospitalizations Through Month 42 in Transthyretin Amyloid Cardiomyopathy Across All Pre-specified Patient SubgroupsStern Lily, Fine Nowell, Maurer Mathew, Grogan Martha, Ambardekar Amrut, Grodin Justin, Soman Prem, Garcia-pavia Pablo, Chen Chris, Siddhanti Suresh, Tamby Jean-francois, Fox Jonathan