Final ID: 4363731
Interferon-inducible macrophages aggravate fulminant myocarditis via ACOD1-mediated mitochondrial dysfunction
Abstract Body (Do not enter title and authors here): Background
Fulminant myocarditis (FM) is a life-threatening inflammatory cardiac disorder characterized by rapid clinical deterioration and systemic cytokine storm, yet the underlying immune cell heterogeneity and mechanisms remain incompletely defined.
Research Hypothesis
We hypothesized that specific macrophage subsets drive systemic inflammation and cardiac injury in FM through interferon-regulated mitochondrial signaling pathways.
Methods
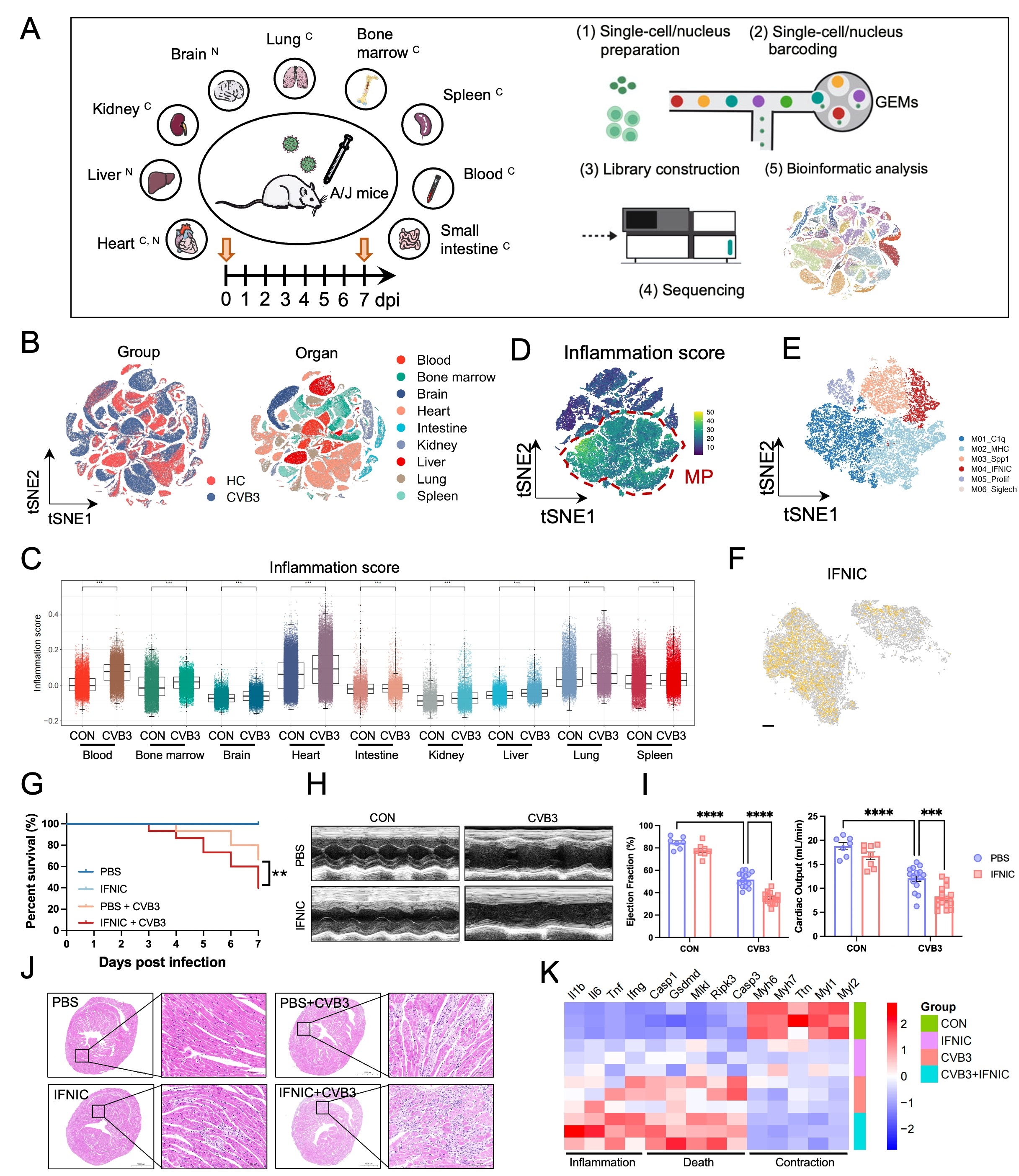
We performed multi-organ single-cell and single-nucleus RNA sequencing (sc/snRNA-seq) across nine organs in coxsackievirus B3 (CVB3)-induced FM murine models, complemented by spatial transcriptomics in FM patient cardiac biopsies. Functional studies were conducted using interferon-inducible macrophages (IFNIC)-tracing mice, rAAV-mediated gene modulation, and in vitro assays with CVB3-stimulated macrophages.
Results
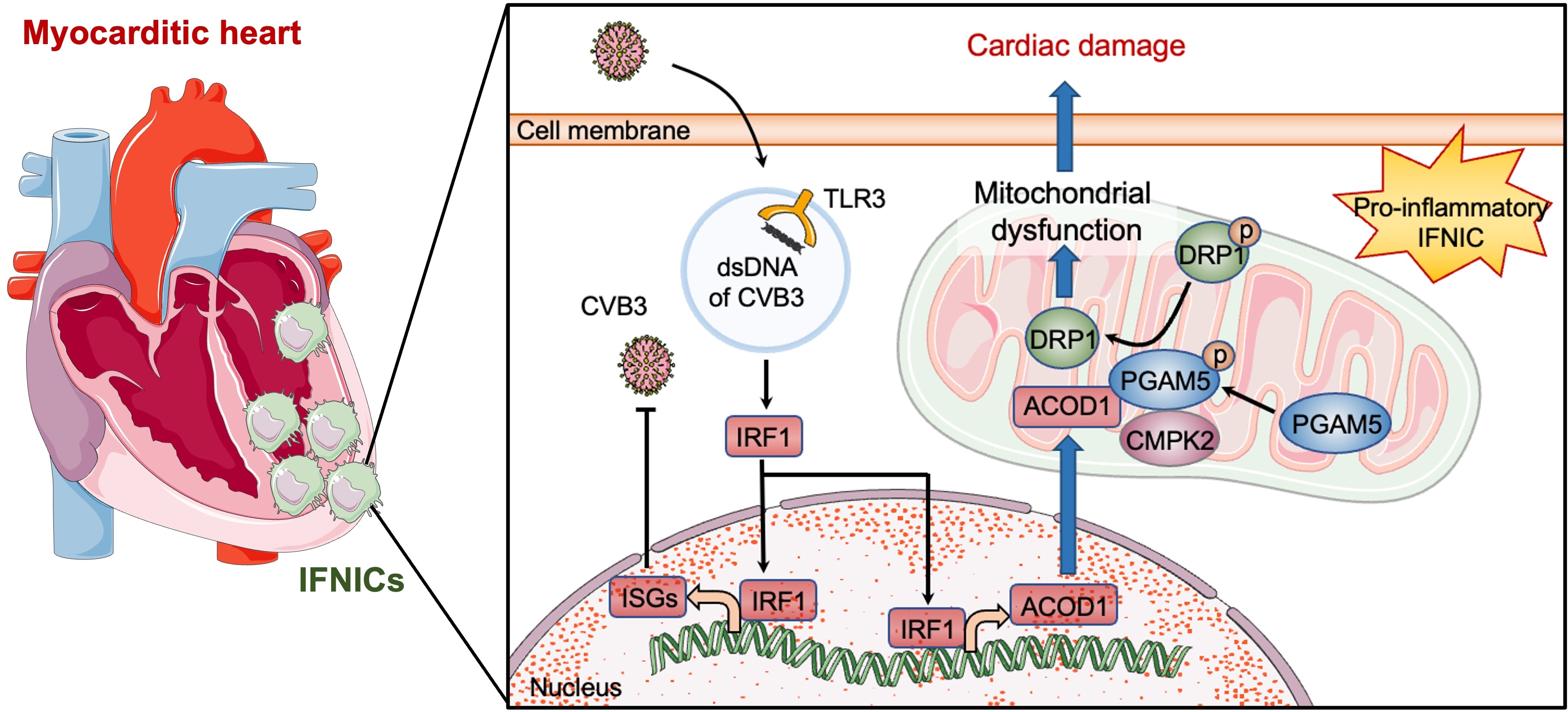
scRNA-seq revealed widespread immune activation across multiple organs, with the heart as the primary site of CVB3 infection and cytokine release. A distinct macrophage subcluster, termed interferon-inducible macrophages (IFNIC), markedly accumulated in the FM heart of both mice and patients. IFNICs expressed elevated inflammatory cytokines and were characterized by enrichment of the interferon-responsive gene ACOD1. IFNIC adoptive transfer exacerbated cardiac dysfunction and mortality in FM mice. Mechanistically, CVB3 triggered TLR3–IRF1 signaling in macrophages, transcriptionally inducing ACOD1. ACOD1 localized to mitochondria and directly interacted with PGAM5 and CMPK2, promoting PGAM5 phosphorylation and enhancing mitochondrial fission. This ACOD1–PGAM5–CMPK2 axis impaired mitochondrial function and amplified macrophage-driven inflammation. Genetic or pharmacologic inhibition of ACOD1 or mitochondrial fission improved survival and cardiac function in FM mice.
Conclusion
Our study identifies IFNICs as key effectors of inflammation in FM and reveals a novel ACOD1-mediated mitochondrial regulatory mechanism. Targeting the TLR3–IRF1–ACOD1–PGAM5 pathway may represent a promising immunometabolic strategy for FM therapy.
Fulminant myocarditis (FM) is a life-threatening inflammatory cardiac disorder characterized by rapid clinical deterioration and systemic cytokine storm, yet the underlying immune cell heterogeneity and mechanisms remain incompletely defined.
Research Hypothesis
We hypothesized that specific macrophage subsets drive systemic inflammation and cardiac injury in FM through interferon-regulated mitochondrial signaling pathways.
Methods
We performed multi-organ single-cell and single-nucleus RNA sequencing (sc/snRNA-seq) across nine organs in coxsackievirus B3 (CVB3)-induced FM murine models, complemented by spatial transcriptomics in FM patient cardiac biopsies. Functional studies were conducted using interferon-inducible macrophages (IFNIC)-tracing mice, rAAV-mediated gene modulation, and in vitro assays with CVB3-stimulated macrophages.
Results
scRNA-seq revealed widespread immune activation across multiple organs, with the heart as the primary site of CVB3 infection and cytokine release. A distinct macrophage subcluster, termed interferon-inducible macrophages (IFNIC), markedly accumulated in the FM heart of both mice and patients. IFNICs expressed elevated inflammatory cytokines and were characterized by enrichment of the interferon-responsive gene ACOD1. IFNIC adoptive transfer exacerbated cardiac dysfunction and mortality in FM mice. Mechanistically, CVB3 triggered TLR3–IRF1 signaling in macrophages, transcriptionally inducing ACOD1. ACOD1 localized to mitochondria and directly interacted with PGAM5 and CMPK2, promoting PGAM5 phosphorylation and enhancing mitochondrial fission. This ACOD1–PGAM5–CMPK2 axis impaired mitochondrial function and amplified macrophage-driven inflammation. Genetic or pharmacologic inhibition of ACOD1 or mitochondrial fission improved survival and cardiac function in FM mice.
Conclusion
Our study identifies IFNICs as key effectors of inflammation in FM and reveals a novel ACOD1-mediated mitochondrial regulatory mechanism. Targeting the TLR3–IRF1–ACOD1–PGAM5 pathway may represent a promising immunometabolic strategy for FM therapy.
More abstracts on this topic:
A Heart Transplant Patient’s Mysterious Illness: A Diagnostic Odyssey
Alkalbani Mutaz, Nayer Hassan, Cochrane Adam, Saeed Ibrahim, Psotka Mitchell, Rollins Allman, Kennedy Jamie, Blumer Vanessa
A Multi-Population-First Approach Leveraging UK Biobank (UKBB) and All of Us (AoU) Datasets Reveals Higher Cardiomyopathy Variant Burden in Individuals with MyocarditisGurumoorthi Manasa, Khanji Mohammed, Munroe Patricia, Petersen Steffen, Landstrom Andrew, Chahal Anwar, Hesse Kerrick, Asatryan Babken, Shah Ravi, Sharaf Dabbagh Ghaith, Wolfe Rachel, Shyam Sundar Vijay, Mohiddin Saidi, Aung Nay