Final ID: MP2391
GLP1 Receptor Agonist Use in Obstructive Sleep Apnea and the Risk of Developing Atrial Fibrillation
Abstract Body (Do not enter title and authors here): Introduction: GLP1 receptor agonists (GLP1-RA) are increasingly prescribed, with many of their potential benefits elucidated in recent studies. Consequently, GLP1-RAs were recently approved for the treatment of obstructive sleep apnea (OSA). There is a known strong association between OSA and atrial fibrillation (AF), with up to 5% of OSA patients developing AF and as many as 50% of AF patients having OSA. In this retrospective study, we analyze a cohort of patients with OSA prescribed GLP1-RAs and assess their risk of developing AF as compared to OSA patients who were not prescribed GLP1-RAs.
Hypothesis: The use of GLP1-RAs will confer a reduced risk of developing AF in OSA patients.
Methods: All Icahn Mount Sinai Health System patients were searched using TriNetX from 1/1/2005-5/31/2025. Patients with OSA, BMI >25, and age >18 years were included. Patients who did not meet the inclusion criteria, those with any arrhythmias prior to the index event, those with central sleep apnea, or those with baseline structural heart disease or heart failure were excluded. The control cohort was composed of included patients who were not prescribed a GLP1-RA. The cohorts were propensity matched and balanced for baseline characteristics (i.e. BMI, hypertension, CPAP device use, age, etc.). Statistical analysis was performed via the Cox regression method using the built-in TriNetX calculator. Outcomes are reported as risk ratios (RR) and 95% confidence intervals (CI).
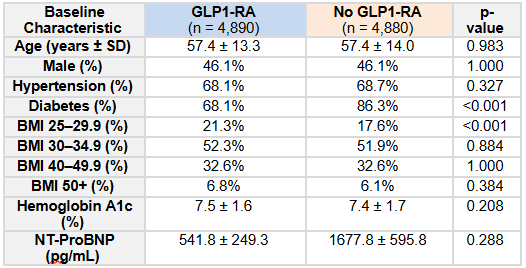
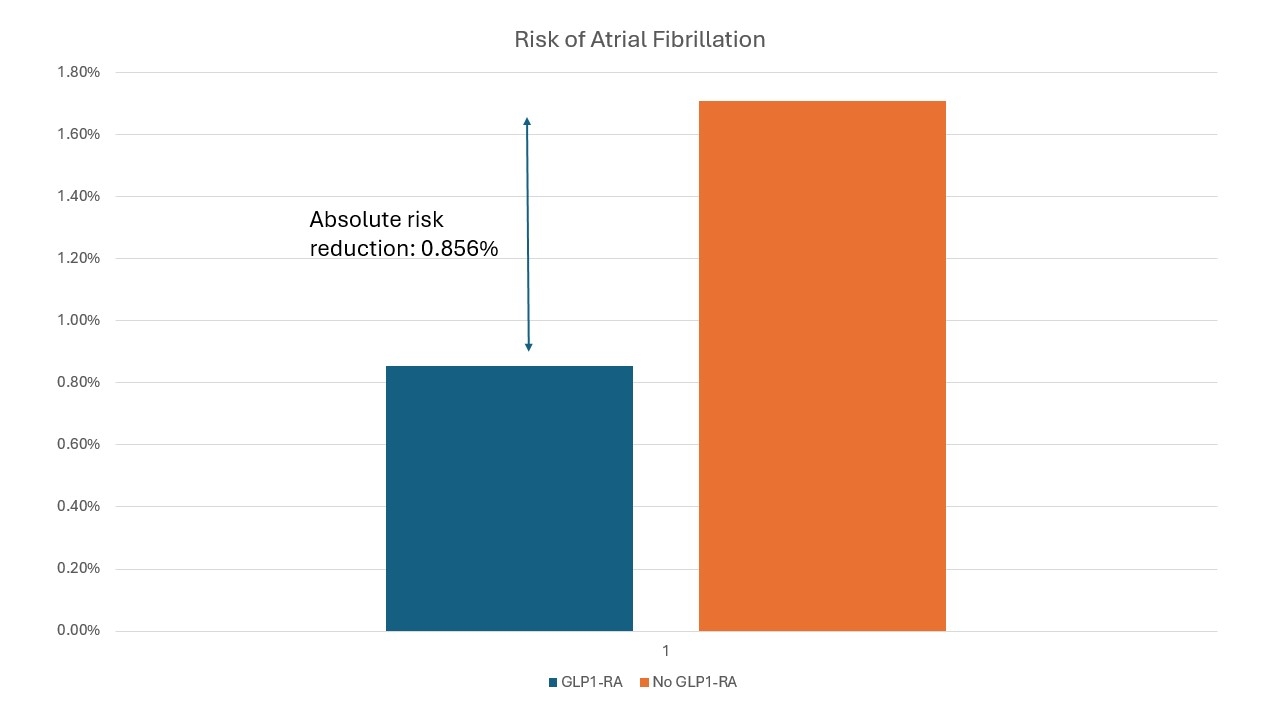
Results: A total of 9,370 patients with OSA were included in the analysis (GLP1 n=4,690; no GLP1 n=4,680). Baseline characteristics of the cohorts are reported in Table 1. The median follow-up from diagnosis of OSA for the non-GLP1-RA group was 958 days. The median follow-up for the GLP1-RA group was 491 days, with a median time from diagnosis of OSA to prescription of GLP1-RA of 380 days (total median follow-up from diagnosis of OSA 871 days). There was a significant 50.1% reduction in the risk ratio of developing AF for OSA patients prescribed GLP1-RAs (RR 0.499, 95% CI 0.342-0.728, P = 0.0002) as compared to those who were not (Figure 1).
Conclusion: GLP1-RAs may confer a significant protective effect on patients with OSA in reducing the risk of developing AF. Further research, including prospective randomized-controlled trials, is needed to elucidate the mechanism of this effect and to determine whether the effect of GLP1-RAs is in proportion to the effect of weight loss experienced by the patients.
Hypothesis: The use of GLP1-RAs will confer a reduced risk of developing AF in OSA patients.
Methods: All Icahn Mount Sinai Health System patients were searched using TriNetX from 1/1/2005-5/31/2025. Patients with OSA, BMI >25, and age >18 years were included. Patients who did not meet the inclusion criteria, those with any arrhythmias prior to the index event, those with central sleep apnea, or those with baseline structural heart disease or heart failure were excluded. The control cohort was composed of included patients who were not prescribed a GLP1-RA. The cohorts were propensity matched and balanced for baseline characteristics (i.e. BMI, hypertension, CPAP device use, age, etc.). Statistical analysis was performed via the Cox regression method using the built-in TriNetX calculator. Outcomes are reported as risk ratios (RR) and 95% confidence intervals (CI).
Results: A total of 9,370 patients with OSA were included in the analysis (GLP1 n=4,690; no GLP1 n=4,680). Baseline characteristics of the cohorts are reported in Table 1. The median follow-up from diagnosis of OSA for the non-GLP1-RA group was 958 days. The median follow-up for the GLP1-RA group was 491 days, with a median time from diagnosis of OSA to prescription of GLP1-RA of 380 days (total median follow-up from diagnosis of OSA 871 days). There was a significant 50.1% reduction in the risk ratio of developing AF for OSA patients prescribed GLP1-RAs (RR 0.499, 95% CI 0.342-0.728, P = 0.0002) as compared to those who were not (Figure 1).
Conclusion: GLP1-RAs may confer a significant protective effect on patients with OSA in reducing the risk of developing AF. Further research, including prospective randomized-controlled trials, is needed to elucidate the mechanism of this effect and to determine whether the effect of GLP1-RAs is in proportion to the effect of weight loss experienced by the patients.
More abstracts on this topic:
Age-stratified Monogenic and Polygenic Contributions for Atrial Fibrillation in the All of Us Research Program
Chen Zhanlin, Gordon Adam, Webster Gregory
A Rare Case of Genetic Cardiomyopathy: SCN5A mutation-associated Multifocal Ectopic Premature Purkinje-related Complexes Syndrome with Heart Failure with Improved Ejection FractionOsei Albert, Howard Ato, Bhonsale Aditya, Hickey Gavin