Final ID: MP1719
A Phase 2 Multicenter, Randomized, Double-Blind, Placebo-Controlled Study of Monthly or Quarterly Subcutaneous Administration of the Interleukin-6 Inhibitor Pacibekitug in Patients With Elevated High-Sensitivity C-Reactive Protein and Chronic Kidney Disease: 90-Day Analyses from TRANQUILITY
Abstract Body (Do not enter title and authors here): Introduction: Existing evidence supports the cardiovascular disease (CVD) therapeutic potential of blocking interleukin-6 (IL-6). Pacibekitug is a long-acting, fully human monoclonal antibody against the IL-6 cytokine. Pacibekitug is being evaluated in a dose-ranging study of patients with elevated high-sensitivity C-reactive protein (hs-CRP) and chronic kidney disease (CKD), a group with established chronic inflammation. Study findings will inform dose selection in subsequent trials of high-risk patients with CVD.
Methods: TRANQUILITY (NCT06362759) is an ongoing, randomized, double-blind, placebo-controlled Phase 2 trial in patients with stage 3 or 4 CKD and hs-CRP ≥2.0 and <15 mg/L. Patients were stratified by CKD stage and randomized to receive subcutaneous pacibekitug 50 mg quarterly, 25 mg quarterly, 15 mg monthly, or placebo for 6 months. The primary endpoint is time-averaged % change from baseline in hs-CRP through Day 90 (primary evaluation period). This prespecified analysis reports the pharmacodynamic and safety data through the interim data extract date.
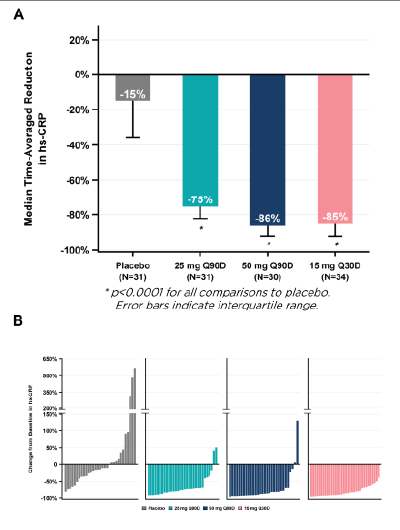
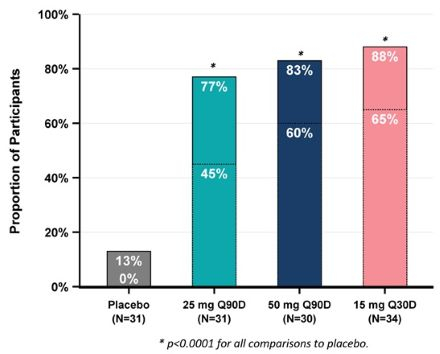
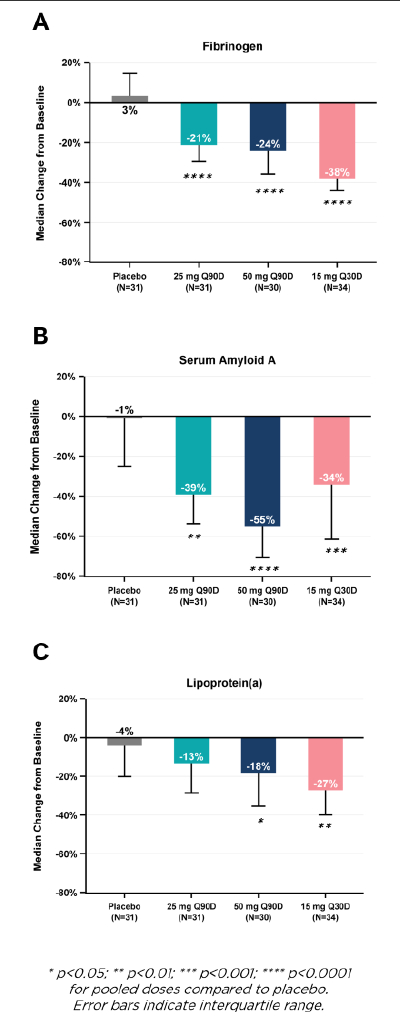
Results: The randomized population consisted of 143 participants. Primary analyses used the modified intention-to-treat set (N=126). Median age was 71 years; 62% were women. Baseline estimated glomerular filtration rate and hs-CRP were 43 ml/min/1.73 m2 and 4.45 mg/L. Median time-averaged % reduction in hs-CRP through Day 90 was 86%, 75%, and 85% for the 50 mg, 25 mg, and 15 mg arms vs. 15% for the placebo arm (all p<0.0001); several patients achieved hs-CRP reductions ≥50% (Figure 1). The percentage of patients with hs-CRP <1 mg/L at Day 90 was 60%, 45%, and 65% for the 50 mg, 25 mg, and 15 mg arms vs. 13% for placebo (all p<0.0001) (Figure 2). Hs-CRP was significantly reduced with pacibekitug across all prespecified groups. Significant reductions in fibrinogen, serum amyloid A, and lipoprotein(a) were observed across pacibekitug arms vs. placebo (Figure 3). Incidence of adverse events (AEs) was 54% and 56% in the pooled pacibekitug and placebo arms. Similar findings were observed for serious AEs (10% vs. 11%), infections (24% vs. 22%), and serious infections (4% vs. 3%).
Conclusions: IL-6 inhibition with quarterly dosing of pacibekitug significantly reduced hs-CRP levels, with no safety signals at 90 days. To our knowledge, pacibekitug is the first IL-6 inhibitor to demonstrate significant and sustained reductions in hs-CRP with quarterly dosing.
Methods: TRANQUILITY (NCT06362759) is an ongoing, randomized, double-blind, placebo-controlled Phase 2 trial in patients with stage 3 or 4 CKD and hs-CRP ≥2.0 and <15 mg/L. Patients were stratified by CKD stage and randomized to receive subcutaneous pacibekitug 50 mg quarterly, 25 mg quarterly, 15 mg monthly, or placebo for 6 months. The primary endpoint is time-averaged % change from baseline in hs-CRP through Day 90 (primary evaluation period). This prespecified analysis reports the pharmacodynamic and safety data through the interim data extract date.
Results: The randomized population consisted of 143 participants. Primary analyses used the modified intention-to-treat set (N=126). Median age was 71 years; 62% were women. Baseline estimated glomerular filtration rate and hs-CRP were 43 ml/min/1.73 m2 and 4.45 mg/L. Median time-averaged % reduction in hs-CRP through Day 90 was 86%, 75%, and 85% for the 50 mg, 25 mg, and 15 mg arms vs. 15% for the placebo arm (all p<0.0001); several patients achieved hs-CRP reductions ≥50% (Figure 1). The percentage of patients with hs-CRP <1 mg/L at Day 90 was 60%, 45%, and 65% for the 50 mg, 25 mg, and 15 mg arms vs. 13% for placebo (all p<0.0001) (Figure 2). Hs-CRP was significantly reduced with pacibekitug across all prespecified groups. Significant reductions in fibrinogen, serum amyloid A, and lipoprotein(a) were observed across pacibekitug arms vs. placebo (Figure 3). Incidence of adverse events (AEs) was 54% and 56% in the pooled pacibekitug and placebo arms. Similar findings were observed for serious AEs (10% vs. 11%), infections (24% vs. 22%), and serious infections (4% vs. 3%).
Conclusions: IL-6 inhibition with quarterly dosing of pacibekitug significantly reduced hs-CRP levels, with no safety signals at 90 days. To our knowledge, pacibekitug is the first IL-6 inhibitor to demonstrate significant and sustained reductions in hs-CRP with quarterly dosing.
More abstracts on this topic:
A Phase 2a randomized controlled trial of once-daily versus twice-daily remote ischemic conditioning in vascular cognitive impairment (TRIC-VCI)
Ganesh Aravind, Mccreary Cheryl, Sahlas Demetrios, Sharma Mukul, Swartz Richard, Smith Eric, Barber Philip, Black Sandra, Corbett Dale, Field Thalia, Frayne Richard, Hachinski Vladimir, Ismail Zahinoor, Mai Lauren
A Framework for Developing Prehospital Intracerebral Hemorrhage Recognition Scales and TechnologiesTaleb Shayandokht, Hsu Jamie, Saver Jeffrey