Final ID: MDP475
Triglyceride Levels Associate With Cardiovascular Risk Across the Full Range but Not Among Individuals With Mild-to-Moderate Hypertriglyceridemia
Guidelines focus on individuals with triglyceride levels of 200 to 499 mg/dL (2.3 and 5.6 mmol/L), a group for whom triglyceride-lowering therapy does not convincingly decrease risk.
Hypotheses and goals
We re-assessed the hypotheses that triglyceride levels across the full biological range and within this constrained range strongly associate with cardiovascular risk.
Methods
We calculated multivariable adjusted hazard ratios for major cardiovascular events and death according to baseline triglyceride levels among 119,573 individuals with triglycerides across the full biological range from the Copenhagen General Population Study, among 27,757 individuals with baseline triglycerides between 200 and 499 mg/dL (2.3 and 5.6 mmol/L) from the Copenhagen General Population Study and the Women’s Health Study, and among 31,372 individuals with mild-to-moderate hypertriglyceridemia participating in the PROMINENT, REDUCE-IT, and STRENGTH randomized trials.
Results
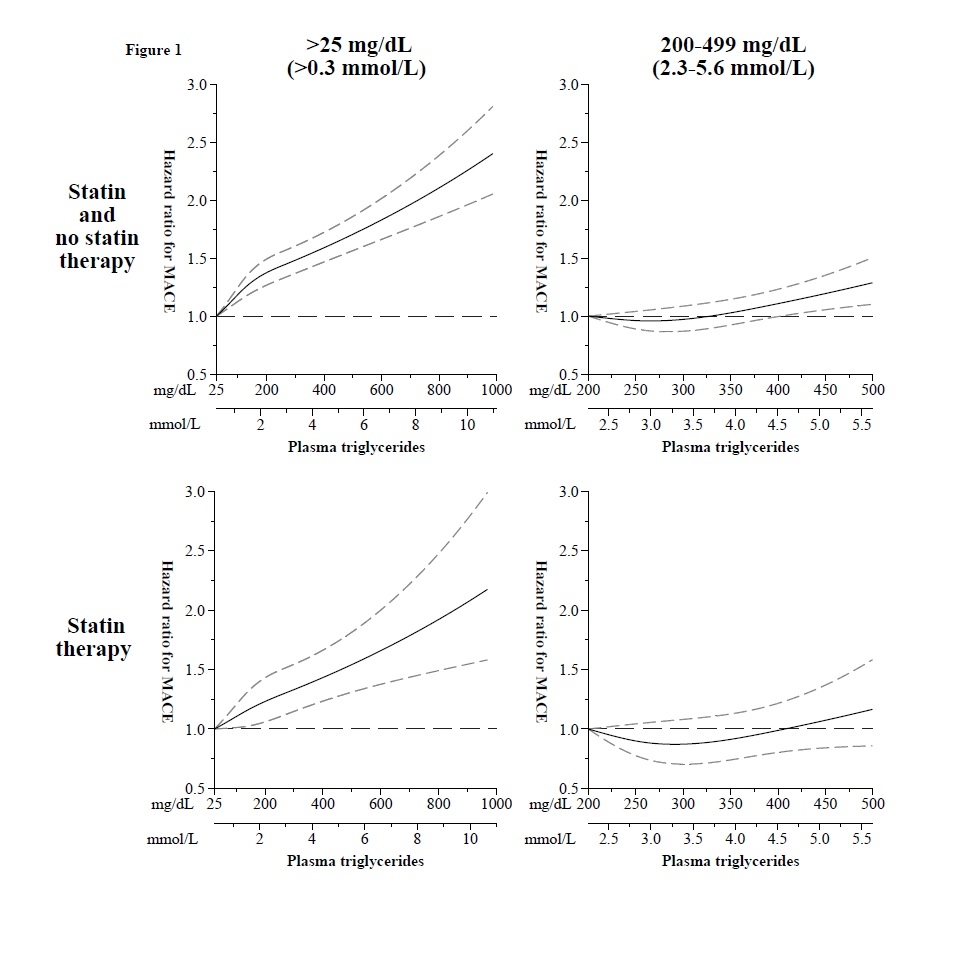
Increasing triglyceride levels across the full range were associated with increasing risk of major cardiovascular events (N=12,241) (Figure 1). In individuals with mild-to-moderate hypertriglyceridemia from the two cohorts, combined hazard ratios (95% confidence interval) for major cardiovascular events (N=3,928) from lowest to highest triglyceride quartile were 1.0 (referent), 0.95 (0.87-1.04), 1.04 (0.95-1.13), and 1.13 (1.04-1.23). In the contemporary trials of patients selected for mild-to-moderate hypertriglyceridemia, the corresponding hazard ratios for major cardiovascular events (N=4,265) from lowest to highest triglyceride quartile were 1.0 (referent), 1.01 (0.93-1.10), 1.05 (0.96-1.14), and 1.10 (1.01-1.19). In neither prospective cohorts nor trials were triglyceride levels strongly associated with risk of cardiovascular or all-cause death within the constrained range.
Conclusions
Contrary to expectations, individuals with mild-to-moderate hypertriglyceridemia may not express the same magnitude of cardiovascular risk as that observed across the full range of plasma triglycerides. Future trials of triglyceride-lowering therapy may want to consider enrollment across a wider range of triglyceride levels if there is no prior history of pancreatitis.
- Nordestgaard, Ask ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Nordestgaard, Borge ( Copenhagen University Hospital - Herlev and Gentofte , Herlev , Denmark )
- Ridker, Paul ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Pradhan, Aruna ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Everett, Brendan ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Macfadyen, Jean ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Bhatt, Deepak ( Mount Sinai Fuster Heart Hospital , New York , New York , United States )
- Visseren, Frank ( University Medical Center Utrecht , Utrecht , Netherlands )
- Libby, Peter ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Santos, Raul ( Heart Institute of the University of Sao Paulo (InCor) , Sao Paulo , Brazil )
- Nissen, Steven ( Cleveland Clinic , Cleveland , Ohio , United States )
Meeting Info:
Session Info:
The Crystal Ball of CVD Risk Assessment
Saturday, 11/16/2024 , 02:50PM - 04:15PM
Moderated Digital Poster Session
More abstracts on this topic:
Soheili Fariborz, Almontashiri Naif, Heydarikhorneh Niloufar, Vilmundarson Ragnar, Chen Hsiao-huei, Stewart Alexandre
A Loss-of-Function Missense Variant in ANGPTL3 Exerts Protective Effects Against Kidney Disease RiskZhang David, Ritchie Marylyn, Rader Daniel, Cuchel Marina
More abstracts from these authors:
Sherratt Samuel, Libby Peter, Bhatt Deepak, Mason Preston
Breaking the Cycle: Addressing Residual Inflammatory Risk in ASCVD & CKD with Next-Generation TargetedEverett Brendan, Ridker Paul, Shah Binita