Final ID: MP1080
Gαq-dependent signaling promotes atrial fibrillation through arrhythmogenic triggered calcium waves and inhomogeneous conduction in a canine heart failure model
Abstract Body (Do not enter title and authors here): Background: Together with conduction inhomogeneity (CI) secondary to fibrosis, arrhythmogenic triggered Ca2+ waves (TCW) are a hallmark of atrial electrophysiology in heart failure (HF). TCW promote atrial fibrillation (AF) by prolonging the action potential duration, thus favoring reentry. Endothelin-1 (ET-1) and downstream protein Gαq are markedly upregulated in the atria of patients with HF. ET-1-Gαq signaling was shown to affect both Ca2+ signaling and fibrosis. We thus hypothesize that ET-1-Gαq signaling contributes to AF in HF through a dual mechanism, by promoting TCW and CI.
Hypothesis: Inhibition of atrial ET-1-Gαq signaling with Gαq inhibitory peptides (Gαq-ct) attenuates AF inducibility and CI in a HF model, and TCW in isolated atrial myocytes.
Methods: For in vivo EP, plasmids expressing Gαq-ct (or scrambled sequence) were injected and electroporated epicardially in the canine left atrium (n=5 for Gαq-ct and 10 for control). HF was then induced by ventricular tachypacing (VTP) for 3 weeks. At terminal EP study, left atrial conduction vectors were recorded with high-density mapping and AF inducibility assessed. Atrial tissue was cryopreserved and fibrosis quantified by Masson Trichrome. For in vitro EP, atrial myocytes were isolated from normal animals or after induction of HF by 3 weeks of VTP (n=4 for each). Myocytes were treated with cell-permeable Gαq-ct (cp-Gαq-ct) and/or ET-1 prior to confocal line-scan Ca2+ imaging with a Ca2+ sensitive dye and field stimulation at cycle lengths 1000ms to 200ms.
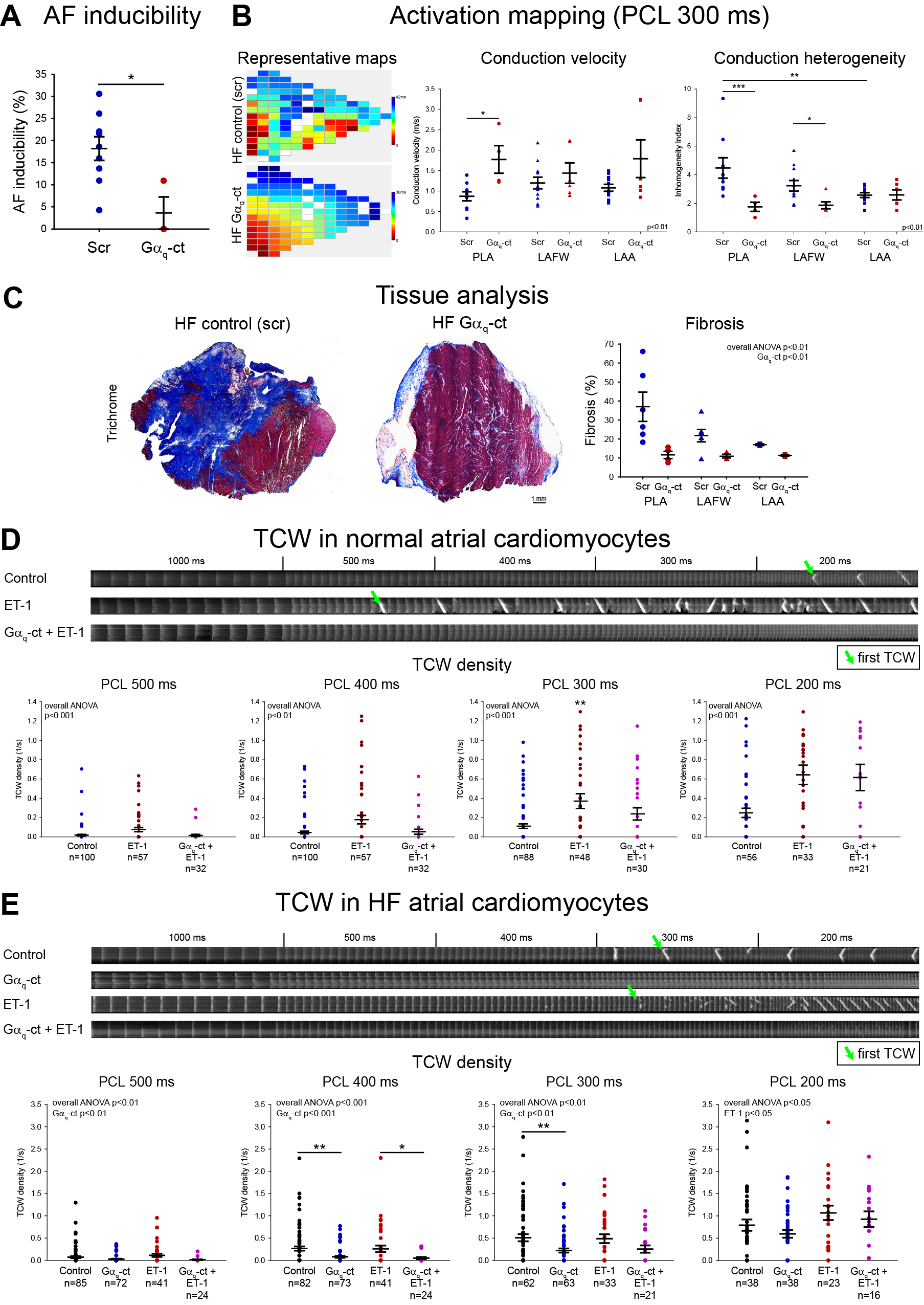
Results: All animals developed clinical HF and LV systolic dysfunction by echocardiogram (LVEF 18.4±3.4% in Gαq-ct, 18.6±3.2% in controls, p=0.97). AF was less inducible in Gαq-ct animals than in controls (p<0.05, Fig. A) and CI was reduced in Gαq-ct animals (p<0.001, Fig. B) which corresponded to an attenuation in atrial fibrosis, predominantly in the posterior left atrium (Fig. C). In normal atrial myocytes, ET-1 significantly increased the density of TCW (Fig. D). This was attenuated by cp-Gαq-ct. HF atrial myocytes had a higher density of TCW, as previously shown. While exposure to ET-1 only marginally increased TCW incidence, cp-Gαq-ct reduced both baseline and ET-1-induced TCW (Fig. E).
Conclusion: ET-1-Gαq-signaling promotes AF in HF through a dual mechanism, by increasing arrhythmogenic TCW and promoting CI. Future optimization of gene therapy targeting ET-1 signaling may lead to novel, mechanism-guided treatments for AF in the context of HF.
Hypothesis: Inhibition of atrial ET-1-Gαq signaling with Gαq inhibitory peptides (Gαq-ct) attenuates AF inducibility and CI in a HF model, and TCW in isolated atrial myocytes.
Methods: For in vivo EP, plasmids expressing Gαq-ct (or scrambled sequence) were injected and electroporated epicardially in the canine left atrium (n=5 for Gαq-ct and 10 for control). HF was then induced by ventricular tachypacing (VTP) for 3 weeks. At terminal EP study, left atrial conduction vectors were recorded with high-density mapping and AF inducibility assessed. Atrial tissue was cryopreserved and fibrosis quantified by Masson Trichrome. For in vitro EP, atrial myocytes were isolated from normal animals or after induction of HF by 3 weeks of VTP (n=4 for each). Myocytes were treated with cell-permeable Gαq-ct (cp-Gαq-ct) and/or ET-1 prior to confocal line-scan Ca2+ imaging with a Ca2+ sensitive dye and field stimulation at cycle lengths 1000ms to 200ms.
Results: All animals developed clinical HF and LV systolic dysfunction by echocardiogram (LVEF 18.4±3.4% in Gαq-ct, 18.6±3.2% in controls, p=0.97). AF was less inducible in Gαq-ct animals than in controls (p<0.05, Fig. A) and CI was reduced in Gαq-ct animals (p<0.001, Fig. B) which corresponded to an attenuation in atrial fibrosis, predominantly in the posterior left atrium (Fig. C). In normal atrial myocytes, ET-1 significantly increased the density of TCW (Fig. D). This was attenuated by cp-Gαq-ct. HF atrial myocytes had a higher density of TCW, as previously shown. While exposure to ET-1 only marginally increased TCW incidence, cp-Gαq-ct reduced both baseline and ET-1-induced TCW (Fig. E).
Conclusion: ET-1-Gαq-signaling promotes AF in HF through a dual mechanism, by increasing arrhythmogenic TCW and promoting CI. Future optimization of gene therapy targeting ET-1 signaling may lead to novel, mechanism-guided treatments for AF in the context of HF.
More abstracts on this topic:
A Bridge from Sweet to Sour: A Case of Recurrent Myocardial Stunning in Diabetic Ketoacidosis
Satish Vikyath, Pargaonkar Sumant, Slipczuk Leandro, Schenone Aldo, Maliha Maisha, Chi Kuan Yu, Sunil Kumar Sriram, Borkowski Pawel, Vyas Rhea, Rodriguez Szaszdi David Jose Javier, Kharawala Amrin, Seo Jiyoung
A-band titin-truncating variant promotes the development of arrhythmia-induced cardiomyopathy in a novel genetically-engineered porcine modelLee Kwonjae, Del Rio Carlos, Mcnally Elizabeth, Pfenniger Anna, Bhatnagar Ashita, Glinton Kristofor, Burrell Amy, Ober Rebecca, Mcluckie Alicia, Bishop Brian, Rogers Christopher, Geist Gail