Final ID: MP975
Vutrisiran Improved Outcomes Versus Placebo in Patients with Transthyretin Amyloidosis with Cardiomyopathy and Severe Chronic Kidney Disease: Post Hoc Analysis of HELIOS-B
Aims: To assess the potential impact of vutrisiran on renal function and efficacy/safety of vutrisiran in pts who advanced to CKD Stage 4 during the HELIOS-B double-blind (DB) period.
Methods: HELIOS-B eligibility criteria included eGFR ≥30 mL/min/1.73m2. Pts were randomized 1:1 to vutrisiran 25 mg or PBO Q3M. In this post hoc analysis of the DB period (up to 33–36 months), the proportion of pts with eGFR decline ≥40% from baseline was assessed. The primary composite endpoint of ACM and CV events, as well as ACM, CV events, and safety, were also assessed in pts who progressed to CKD Stage 4 (eGFR <30 mL/min/1.73m2) during the DB period. Outcomes were assessed overall and by baseline tafamidis use (monotherapy and baseline tafamidis subgroups).
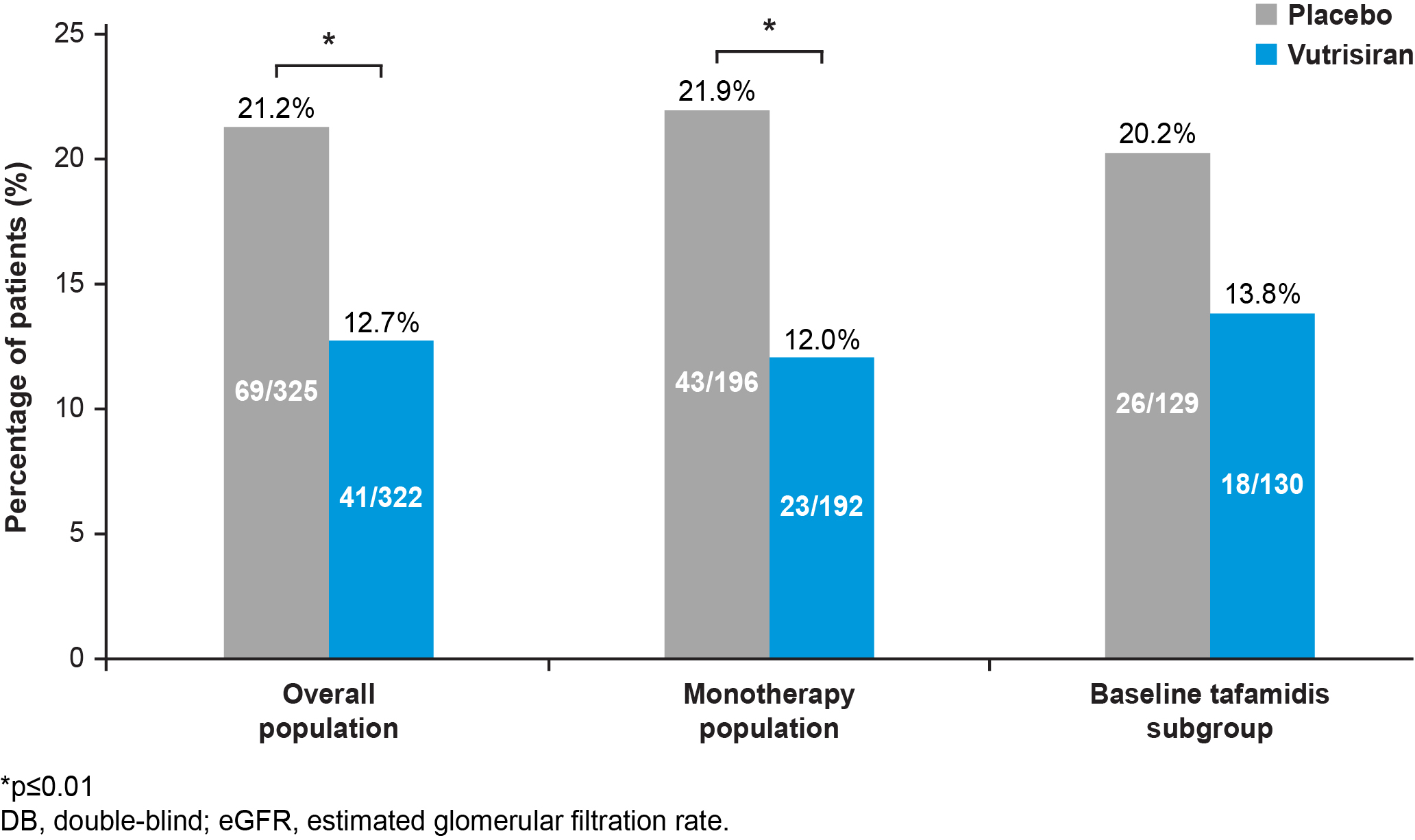
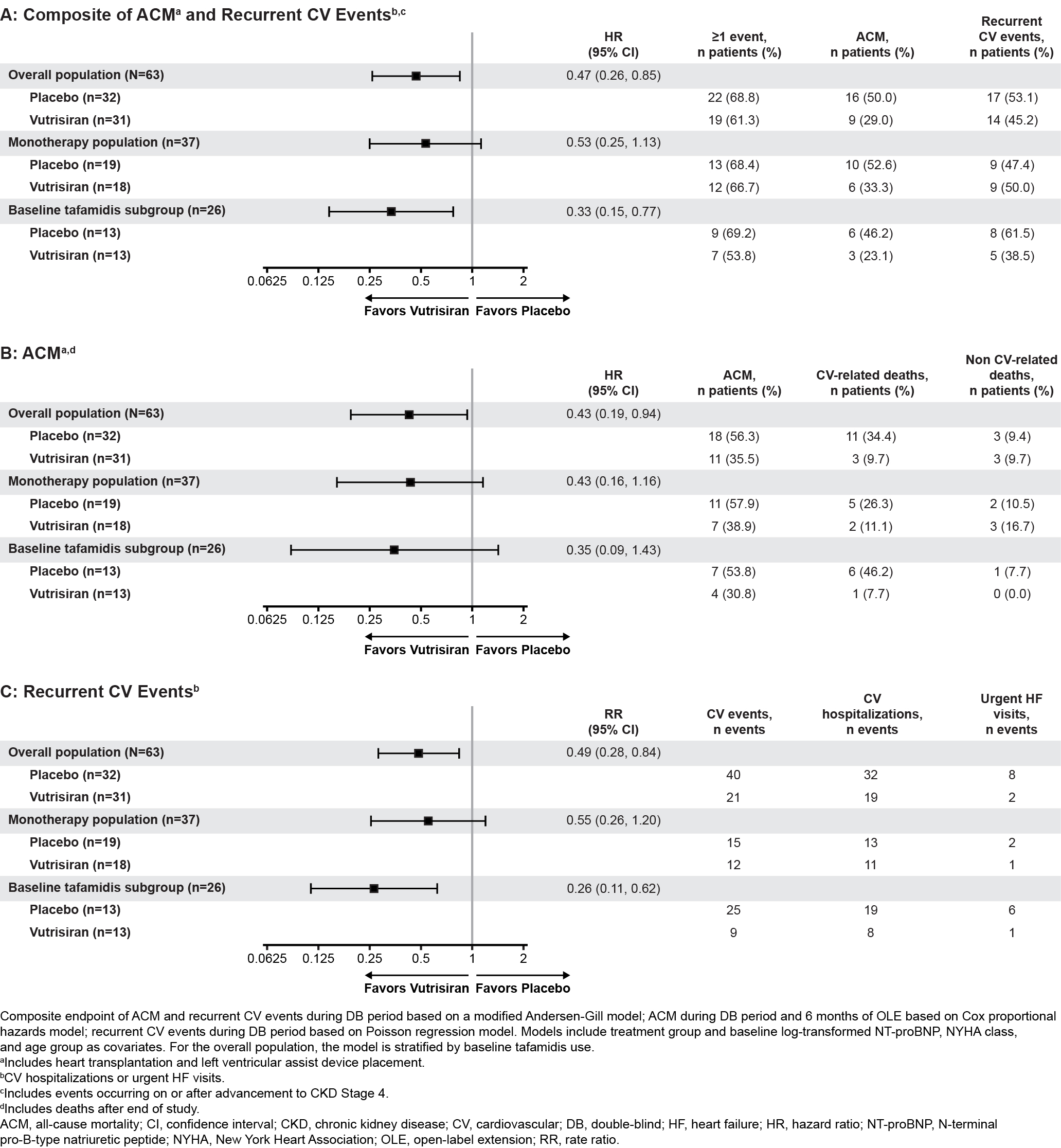
Results: Median (IQR) eGFR at baseline in pts receiving vutrisiran and PBO was 64 (50–81) and 65 (53–81) mL/min/1.73m2, respectively. In the overall population, fewer pts in the vutrisiran group experienced a ≥40% decline in eGFR from baseline vs PBO (12.7% vs 21.2%, respectively); results were consistent in monotherapy and baseline tafamidis subgroups (Figure 1). Among pts who advanced to CKD Stage 4, in the overall population, vutrisiran reduced the risk of composite ACM and CV events vs PBO (HR [95% CI] 0.47 [0.26, 0.85]); similar results were seen in ACM and CV events, separately, and in the monotherapy and baseline tafamidis subgroups (Figure 2; CV-related death: 34.3% with PBO; 9.7% with vutrisiran in the overall population). The safety profile of vutrisiran in pts who advanced to CKD Stage 4 was comparable with PBO. No new safety signals were reported.
Conclusion: Vutrisiran appeared to preserve renal function in pts with ATTR-CM. Consistent with results from the overall population, vutrisiran reduced the risk of ACM and CV events vs PBO in pts with ATTR-CM and advanced CKD in HELIOS-B; results require corroboration in a larger pt population.
- Sheikh, Farooq ( MedStar Heart and Vascular Institute/Georgetown University School of Medicine , Washington , District of Columbia , United States )
- Eraly, Satish ( Alnylam Pharmaceuticals , Cambridge , Massachusetts , United States )
- Moffitt, Colleen ( Alnylam Pharmaceuticals , Cambridge , Massachusetts , United States )
- Yilmaz, Ali ( Klinik für Kardiologie I, Sektion für Herzbildgebung, Universitätsklinikum Münster , Münster , Germany )
- Dang, Julien ( Assistance Publique des Hôpitaux de Paris, Nephrology Department, Hôpital Ambroise Paré , Boulogne-Billancourt , France )
- Fontana, Marianna ( University College London , London , United Kingdom )
- Audard, Vincent ( Assistance Publique des Hôpitaux de Paris, Nephrology Department, Henri Mondor Hospital University, University Paris Est Créteil and National Institute of Health and Medical Research , Créteil , France )
- Garcia-pavia, Pablo ( Hospital Universitario Puerta de Hierro , Madrid , Spain )
- Khouri, Michel ( Duke University School of Medicine , Durham , North Carolina , United States )
- Jobbe-duval, Antoine ( Médipôle Hôpital Mutualiste , Villeurbanne , France )
- Brailovsky, Yevgeniy ( Columbia University Irving Medical Center , New York , New York , United States )
- Zheng, Hua ( Alnylam Pharmaceuticals , Cambridge , Massachusetts , United States )
Meeting Info:
Session Info:
Heart Failure and Cardiomyopathy: From Bench to Bedside
Saturday, 11/08/2025 , 03:15PM - 04:25PM
Moderated Digital Poster Session
More abstracts on this topic:
Muthukkumar Rashmi, Holmes Taylor, Friede Kevin
Association of Albuminuria with Cognition in Midlife: The CARDIA studyChang Ning-shan, Vivek Sithara, Yaffe Kristine, Guan Weihua, Launer Lenore, Seegmiller Jesse, Schreiner Pamela, Sedaghat Sanaz, Shlipak Michael, Jacobs David
More abstracts from these authors:
Alexander Kevin, Garcia-pavia Pablo
Impact of vutrisiran on outpatient worsening heart failure in patients with transthyretin amyloidosis with cardiomyopathy in the HELIOS-B trialFontana Marianna, Maurer Mathew, Gillmore Julian, Bender Shaun, Aldinc Emre, Eraly Satish, Jay Patrick, Solomon Scott