Final ID: MP860
Sodium-Glucose Cotransporter-2 Inhibitors in Moderate Aortic Stenosis
Abstract Body (Do not enter title and authors here): Introduction
Moderate AS is associated with an increased risk of both CV and all-cause mortality. There are currently no approved medications to slow its progression to severe AS. We assessed the effects of sodium-glucose SGLT2is in patients with moderate AS.
Methods
We conducted a retrospective cohort analysis of de-identified, aggregate patient data from the nationwide TriNetX research network. Patients ≥18 years with TTE-proven native trileaflet moderate AS (AVA 1.0-1.5 cm2) (1/2018-1/2022) were identified and classified into 2 groups: 1) patients never prescribed SLGLT2i and 2) patients with SLGLT2i initiation within 3 months of index TTE. They were followed for up to 3 years. Patients with an eGFR <30 were excluded. Primary outcome was progression to severe AS (AVA < 1.0 cm2). Secondary outcomes included all-cause mortality and acute decompensated heart failure (ADHF). We conducted a tight 1:1 propensity score matching for baseline demographics, comorbidities, medications - including HF GDMT, LVEF, AVA, NT-proBNP, and HbA1c levels.
Results
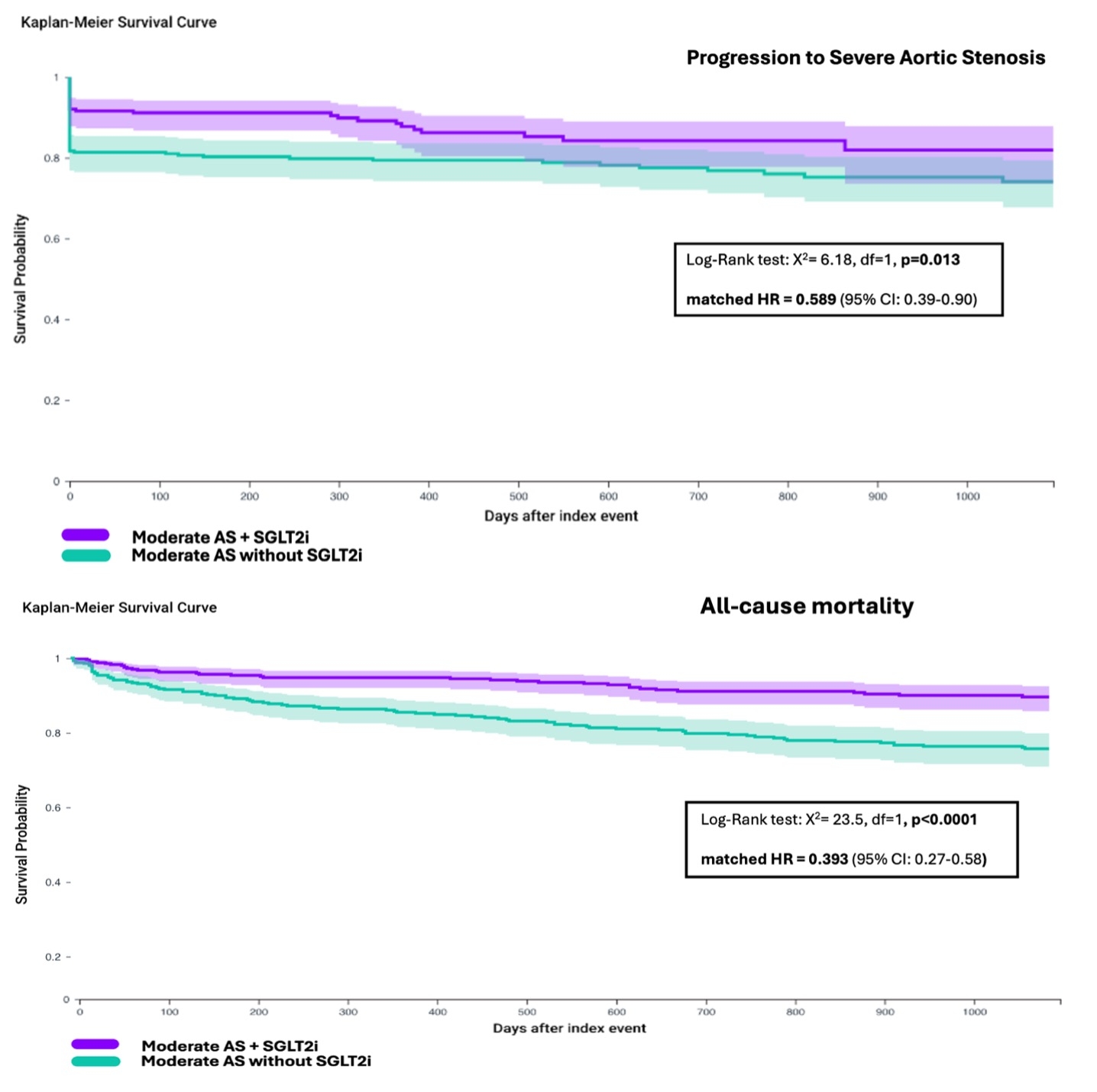
486 patients prescribed an SGLT2i and 3,044 patients never prescribed an SGLT2i were included. Patients in the SGLT2i group were older (71.3 vs. 63.1), more likely to have DM (83% vs. 31%) and CKD (39.3% vs. 23%), and had higher baseline AVA (1.31 cm2 vs. 1.21 cm2; p<0.001). Both groups had similar LVEFs (58.1% vs. 59.3%; p=0.55). During follow-up, patients prescribed SGLT2i were less likely to progress to severe AS (13% vs 22.7% - unadjusted HR 0.548 [95% CI: 0.41–0.73]; p<0.0001). After multivariable adjustment, SGLT2i use was independently associated with reduced risk of progression (aHR 0.553 [95% CI: 0.42–0.73]; p<0.0001). Risk factors for progression were index-TTE AVA, male sex, older age, white race, CAD, HF, and CKD. Following PSM, 620 patients were included (310 per group; mean age 54.3; 55% female; mean AVA 1.27 cm2; mean LVEF 58%). The SGLT2i group was less likely to progress to severe AS (matched HR 0.589 [95% CI: 0.39–0.90]; p=0.013). SGLT2i use was associated with a lower risk of all-cause mortality (mHR 0.393 [95% CI: 0.27–0.58]; p<0.0001) and ADHF (mHR 0.578 [95% CI: 0.34–0.99]; p = 0.04) (Fig 1).
Conclusions
SGLT2is is associated with slower progression from moderate AS to severe AS. Prospective studies are needed to confirm and strengthen these findings.
Moderate AS is associated with an increased risk of both CV and all-cause mortality. There are currently no approved medications to slow its progression to severe AS. We assessed the effects of sodium-glucose SGLT2is in patients with moderate AS.
Methods
We conducted a retrospective cohort analysis of de-identified, aggregate patient data from the nationwide TriNetX research network. Patients ≥18 years with TTE-proven native trileaflet moderate AS (AVA 1.0-1.5 cm2) (1/2018-1/2022) were identified and classified into 2 groups: 1) patients never prescribed SLGLT2i and 2) patients with SLGLT2i initiation within 3 months of index TTE. They were followed for up to 3 years. Patients with an eGFR <30 were excluded. Primary outcome was progression to severe AS (AVA < 1.0 cm2). Secondary outcomes included all-cause mortality and acute decompensated heart failure (ADHF). We conducted a tight 1:1 propensity score matching for baseline demographics, comorbidities, medications - including HF GDMT, LVEF, AVA, NT-proBNP, and HbA1c levels.
Results
486 patients prescribed an SGLT2i and 3,044 patients never prescribed an SGLT2i were included. Patients in the SGLT2i group were older (71.3 vs. 63.1), more likely to have DM (83% vs. 31%) and CKD (39.3% vs. 23%), and had higher baseline AVA (1.31 cm2 vs. 1.21 cm2; p<0.001). Both groups had similar LVEFs (58.1% vs. 59.3%; p=0.55). During follow-up, patients prescribed SGLT2i were less likely to progress to severe AS (13% vs 22.7% - unadjusted HR 0.548 [95% CI: 0.41–0.73]; p<0.0001). After multivariable adjustment, SGLT2i use was independently associated with reduced risk of progression (aHR 0.553 [95% CI: 0.42–0.73]; p<0.0001). Risk factors for progression were index-TTE AVA, male sex, older age, white race, CAD, HF, and CKD. Following PSM, 620 patients were included (310 per group; mean age 54.3; 55% female; mean AVA 1.27 cm2; mean LVEF 58%). The SGLT2i group was less likely to progress to severe AS (matched HR 0.589 [95% CI: 0.39–0.90]; p=0.013). SGLT2i use was associated with a lower risk of all-cause mortality (mHR 0.393 [95% CI: 0.27–0.58]; p<0.0001) and ADHF (mHR 0.578 [95% CI: 0.34–0.99]; p = 0.04) (Fig 1).
Conclusions
SGLT2is is associated with slower progression from moderate AS to severe AS. Prospective studies are needed to confirm and strengthen these findings.
More abstracts on this topic:
A Diagnostic Pitfall: Subclavian Stenosis Mimicking Severe Aortic Stenosis on Echocardiography"
Ezaldin Shady, Abdelsalam Mahmoud, Elsayed Omar, Lee Marciano
Alleviating Aortic Valve Calcification By Blocking TNFα ReceptorsThent Zar Chi, Butcher Jonathan, Zhou Bin