Final ID: MP2767
Macrophage Extracellular Traps Promote Maladaptive Cardiac Remodeling And Heart Failure Via PAD4-dependent Mechanisms
Abstract Body (Do not enter title and authors here):
Background
The activation of inflammatory cells, particularly macrophages, plays a pivotal role in the pathogenesis of cardiac remodeling and failure. However, the precise mechanisms by which macrophages initiate inflammation and orchestrate its resolution remain incompletely understood. It is increasingly recognized that extracellular traps released from immune cells contribute to the progression of various pathologies. However, the significance of macrophage extracellular traps (METs) in heart failure remains to be elucidated.
Aims
The aim of this study was to elucidate the role of METs in heart failure pathogenesis.
Methods
Pressure overload was used to induce heart failure in mice using transverse aortic constriction (TAC) model. Peptidyl arginine deiminase 4 (PAD4) knockout mice were used for in vivo and ex vivo study.
Results
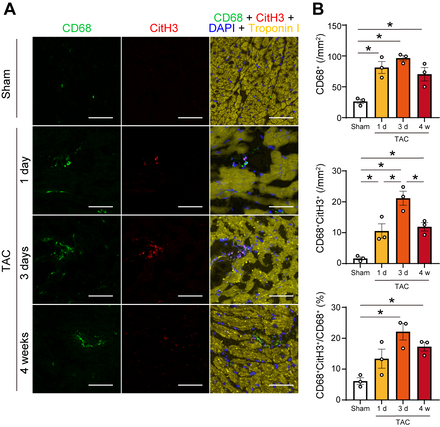
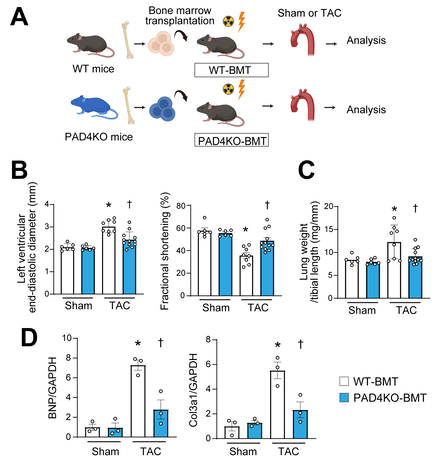
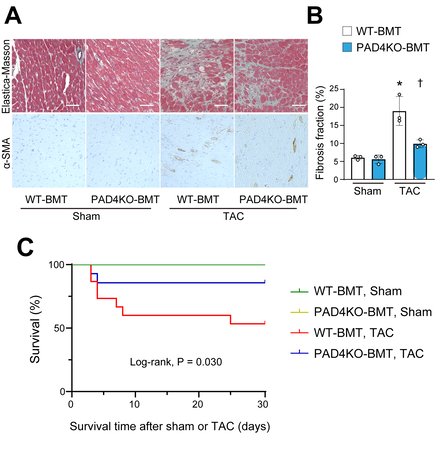
In response to TAC in wild-type mice, METs were identified in myocardial tissue using immunohistochemistry, characterized by structures positive for citrullinated histone H3, CD68, and DAPI (Figure 1). METs were most abundantly observed at 3 days post-TAC and remained detectable throughout the 4-week observation period. In vitro, MET formation was induced in the macrophage cell line following specific stimuli including ionomycin. Given that PAD4 is known to be essential for the process of extracellular traps, neither genetic ablation of PAD4 using CRISPR/Cas9 nor pharmacological inhibition of PAD4 resulted in MET formation. Live-cell imaging confirmed MET formation by bone marrow-derived macrophages from wild-type mice, but not by PAD4-deficient macrophages, indicating that PAD4 activity is indispensable for METs. To determine the in vivo role of METs from bone marrow-derived macrophages, bone marrow transplantation was performed using PAD4 knockout donor mice (Figure 2). Following TAC, recipient mice receiving PAD4-deficient bone marrow exhibited better-preserved cardiac function, reduced myocardial fibrosis, and improved survival compared to those receiving wild-type bone marrow (Figure 3). Ex vivo analyses further demonstrated that conditioned medium containing METs from wild-type macrophages induced fibroblast-to-myofibroblast transition via toll-like receptor 4 signaling.
Conclusion
PAD4-dependent MET formation from bone marrow-derived macrophages represents a novel driver of cardiac remodeling. Targeting MET formation may offer a potential therapeutic strategy for heart failure.
Background
The activation of inflammatory cells, particularly macrophages, plays a pivotal role in the pathogenesis of cardiac remodeling and failure. However, the precise mechanisms by which macrophages initiate inflammation and orchestrate its resolution remain incompletely understood. It is increasingly recognized that extracellular traps released from immune cells contribute to the progression of various pathologies. However, the significance of macrophage extracellular traps (METs) in heart failure remains to be elucidated.
Aims
The aim of this study was to elucidate the role of METs in heart failure pathogenesis.
Methods
Pressure overload was used to induce heart failure in mice using transverse aortic constriction (TAC) model. Peptidyl arginine deiminase 4 (PAD4) knockout mice were used for in vivo and ex vivo study.
Results
In response to TAC in wild-type mice, METs were identified in myocardial tissue using immunohistochemistry, characterized by structures positive for citrullinated histone H3, CD68, and DAPI (Figure 1). METs were most abundantly observed at 3 days post-TAC and remained detectable throughout the 4-week observation period. In vitro, MET formation was induced in the macrophage cell line following specific stimuli including ionomycin. Given that PAD4 is known to be essential for the process of extracellular traps, neither genetic ablation of PAD4 using CRISPR/Cas9 nor pharmacological inhibition of PAD4 resulted in MET formation. Live-cell imaging confirmed MET formation by bone marrow-derived macrophages from wild-type mice, but not by PAD4-deficient macrophages, indicating that PAD4 activity is indispensable for METs. To determine the in vivo role of METs from bone marrow-derived macrophages, bone marrow transplantation was performed using PAD4 knockout donor mice (Figure 2). Following TAC, recipient mice receiving PAD4-deficient bone marrow exhibited better-preserved cardiac function, reduced myocardial fibrosis, and improved survival compared to those receiving wild-type bone marrow (Figure 3). Ex vivo analyses further demonstrated that conditioned medium containing METs from wild-type macrophages induced fibroblast-to-myofibroblast transition via toll-like receptor 4 signaling.
Conclusion
PAD4-dependent MET formation from bone marrow-derived macrophages represents a novel driver of cardiac remodeling. Targeting MET formation may offer a potential therapeutic strategy for heart failure.
More abstracts on this topic:
A Mast Cell-Specific Receptor Mediates Post-Stroke Brain Inflammation Via a Dural-Brain Axis
Kothari Ruchita, Caplan Justin, Gonzalez L. Fernando, Jackson Christopher, Bettegowda Chetan, Huang Judy, Koehler Raymond, Tamargo Rafael, Xu Risheng, Dong Xinzhong, Abdulrahim Mostafa, Oh Hyun Jong, Capuzzi Daniel, Nair Sumil, Zhang Yaowu, Limjunyawong Nathachit, Saini Sarbjit, Kim Jennifer
A Case of Concomitant Wild-Type Transthyretin and Systemic Light Chain Amyloidosis Involving Separate OrgansChiu Leonard, Afrough Aimaz, Nadeem Urooba, Jebakumar Deborah, Grodin Justin