Final ID: MP1072
Cell Painting Based High-Content Phenotypic Screening To Elucidate Kinase Signaling Pathways in BTK-Inhibitor Mediated Atrial Fibrillation
Abstract Body (Do not enter title and authors here): Introduction:
One goal of Precision Cardio-Oncology is to identify and ultimately clinically exploit a fundamental understanding of shared signaling pathways in cardiovascular disease and oncologic processes, such as in BTK inhibitor-mediated atrial fibrillation. In clinical trials, the first-generation BTK inhibitor ibrutinib results in substantially more atrial fibrillation than do next-generation BTK inhibitors.
Purpose:
Here we report the use of high-content imaging (“Cell Painting”) and phenotypic screening of four FDA-approved BTK inhibitors in human iPSC-derived cardiomyocyte models to inform mechanisms underlying this proarrhythmic process.
Methods:
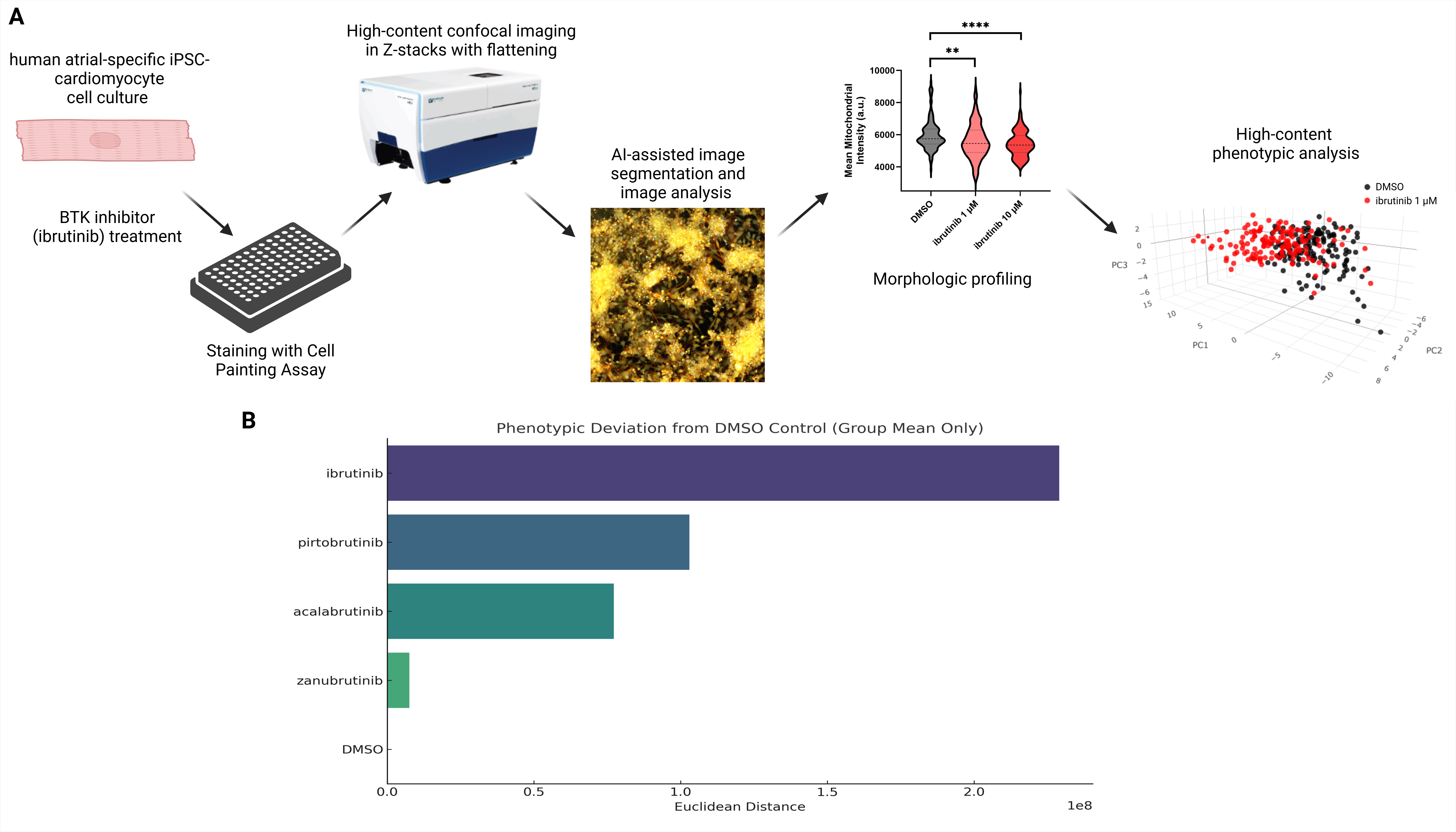
We performed studies using human atrial-specific cardiomyocytes (hiPSC-aCMs) derived from population control induced pluripotent stem cells. Monolayers of hiPSC-aCMs were plated onto 96-well plates and treated with ibrutinib, acalabrutinib, zanubrutinib, pirtobrutinib, or DMSO vehicle control. After 72 hours of treatment, in situ staining with the Cell Painting assay was performed. The method labels subcellular components (e.g. mitochondria, nuclei, endoplasmic reticulum, and cytoskeleton) by simultaneously staining each with a different fluorescent dye. Measurements of mitochondrial intensity, texture, shape, size, and relation to neighboring cellular structures were analyzed for each treatment using an AI-based platform.
Results:
Treatment of hiPSC-aCMs with BTK inhibitors resulted in changes in 65 parameters of mitochondrial structure, including changes in mitochondrial intensity, texture, shape, size, and relation to neighboring cellular structures. 3D-principal component analysis (PCA) of all 65 measured mitochondrial parameters showed significant differences (p<0.0001) in mitochondrial structure between ibrutinib and vehicle-treated hiPSC-aCMs (Figure 1A). Phenotypic deviation scores for all four BTK inhibitors were calculated, with only ibrutinib meeting statistical significance (p<0.001) (Figure 1B).
Conclusions:
Exposure to ibrutinib, but not next-generation BTK inhibitors, causes marked changes in multiple indices of mitochondrial structure, implicating altered mitochondrial function in the development of ibrutinib-related AF. Decreased mitochondrial oxidative phosphorylation and gene expression have been previously shown in hiPSC-aCMs exposed to ibrutinib, providing further biological relevance to these observed phenotypic changes.
One goal of Precision Cardio-Oncology is to identify and ultimately clinically exploit a fundamental understanding of shared signaling pathways in cardiovascular disease and oncologic processes, such as in BTK inhibitor-mediated atrial fibrillation. In clinical trials, the first-generation BTK inhibitor ibrutinib results in substantially more atrial fibrillation than do next-generation BTK inhibitors.
Purpose:
Here we report the use of high-content imaging (“Cell Painting”) and phenotypic screening of four FDA-approved BTK inhibitors in human iPSC-derived cardiomyocyte models to inform mechanisms underlying this proarrhythmic process.
Methods:
We performed studies using human atrial-specific cardiomyocytes (hiPSC-aCMs) derived from population control induced pluripotent stem cells. Monolayers of hiPSC-aCMs were plated onto 96-well plates and treated with ibrutinib, acalabrutinib, zanubrutinib, pirtobrutinib, or DMSO vehicle control. After 72 hours of treatment, in situ staining with the Cell Painting assay was performed. The method labels subcellular components (e.g. mitochondria, nuclei, endoplasmic reticulum, and cytoskeleton) by simultaneously staining each with a different fluorescent dye. Measurements of mitochondrial intensity, texture, shape, size, and relation to neighboring cellular structures were analyzed for each treatment using an AI-based platform.
Results:
Treatment of hiPSC-aCMs with BTK inhibitors resulted in changes in 65 parameters of mitochondrial structure, including changes in mitochondrial intensity, texture, shape, size, and relation to neighboring cellular structures. 3D-principal component analysis (PCA) of all 65 measured mitochondrial parameters showed significant differences (p<0.0001) in mitochondrial structure between ibrutinib and vehicle-treated hiPSC-aCMs (Figure 1A). Phenotypic deviation scores for all four BTK inhibitors were calculated, with only ibrutinib meeting statistical significance (p<0.001) (Figure 1B).
Conclusions:
Exposure to ibrutinib, but not next-generation BTK inhibitors, causes marked changes in multiple indices of mitochondrial structure, implicating altered mitochondrial function in the development of ibrutinib-related AF. Decreased mitochondrial oxidative phosphorylation and gene expression have been previously shown in hiPSC-aCMs exposed to ibrutinib, providing further biological relevance to these observed phenotypic changes.
More abstracts on this topic:
A Curious Complete Heart Block with Carfilzomib
Shah Mohammed, Rahman Naveed, Al-mohamad Talal, Batra Sejal, Vyas Apurva
Alfa-tubulin detyrosination causes mitochondrial dysfunction through suppression of Parkin-mediated mitophagy linking to heart failure with preserved ejection fractionMiura Shunsuke, Nakazato Kazuhiko, Ishida Takafumi, Takeishi Yasuchika, Sekine Toranosuke, Ogawara Ryo, Ichimura Shohei, Yokokawa Tetsuro, Misaka Tomofumi, Oikawa Masayoshi, Kobayashi Atsushi, Yamaki Takayoshi