Final ID: Su2117
Title: Protein interaction profiling of the Kv11.1 potassium channel reveals new therapeutic targets for Long QT Syndrome
Abstract Body (Do not enter title and authors here): Background: The voltage-gated potassium ion channel KV11.1 plays a crucial role in cardiac repolarization. Genetic variants in KV11.1 lead to Long QT Syndrome (LQTS), a condition associated with fatal arrhythmias. Approximately 90% of missense variants linked to LQTS cause intracellular protein transport (trafficking) dysfunction. Some KV11.1 channel blockers act as chemical chaperones (e.g., E-4031) and increase KV11.1 channel trafficking. However, these drugs cannot be used in LQTS patients. The underlying mechanisms, such as the protein networks involved in folding and quality control for KV11.1 channel trafficking remain largely unexplored.
Methods: We used affinity-purification (e.g., coimmunoprecipitation) coupled with mass spectrometry to quantify protein interaction changes in human embryonic kidney cells expressing wild-type KV11.1 or two trafficking-deficient channel variants in the presence or absence of chemical chaperone E-4031. After co-immunoprecipitations and protein digestions, peptides were identified using multiplexed mass spectrometry.
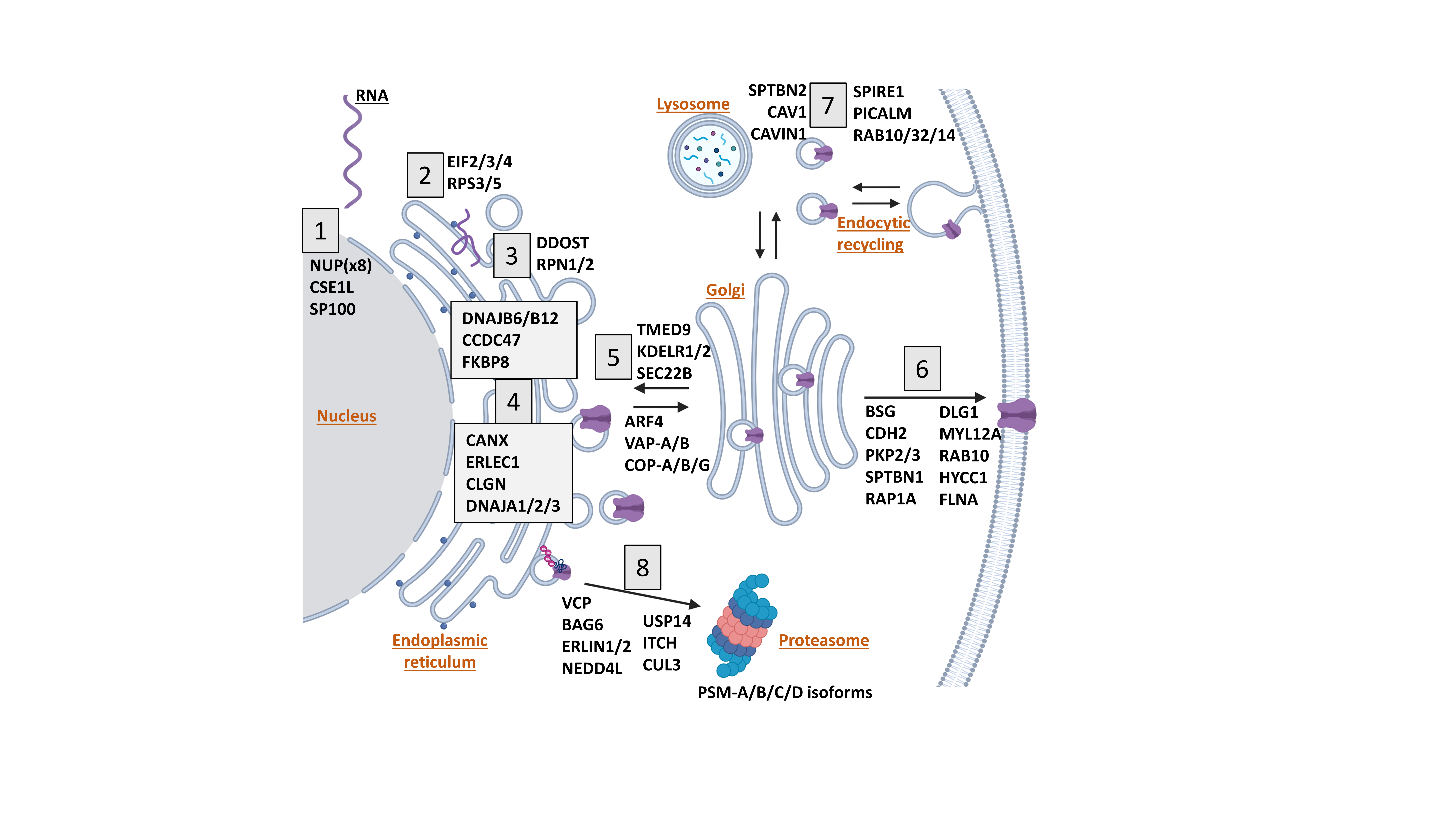
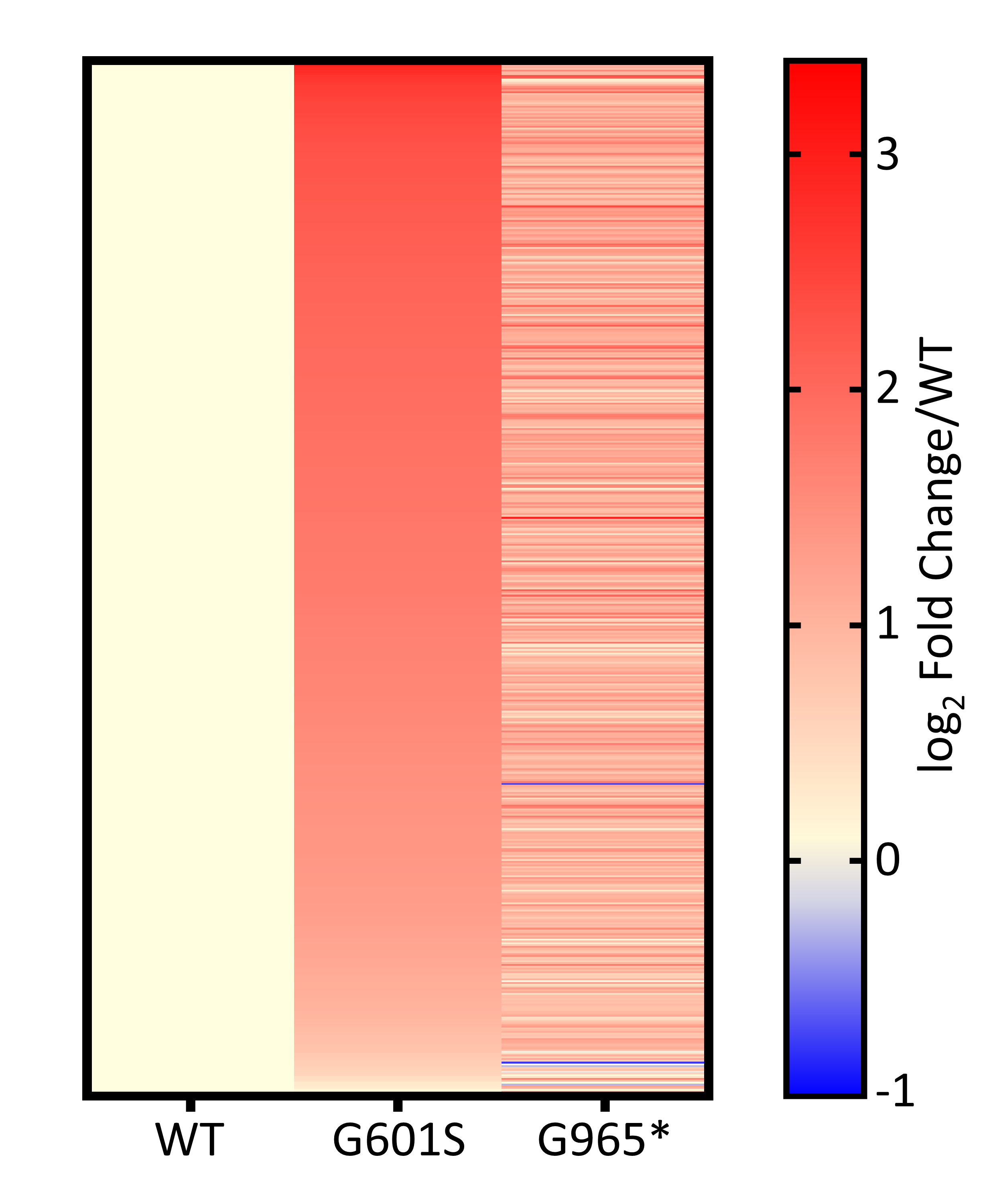
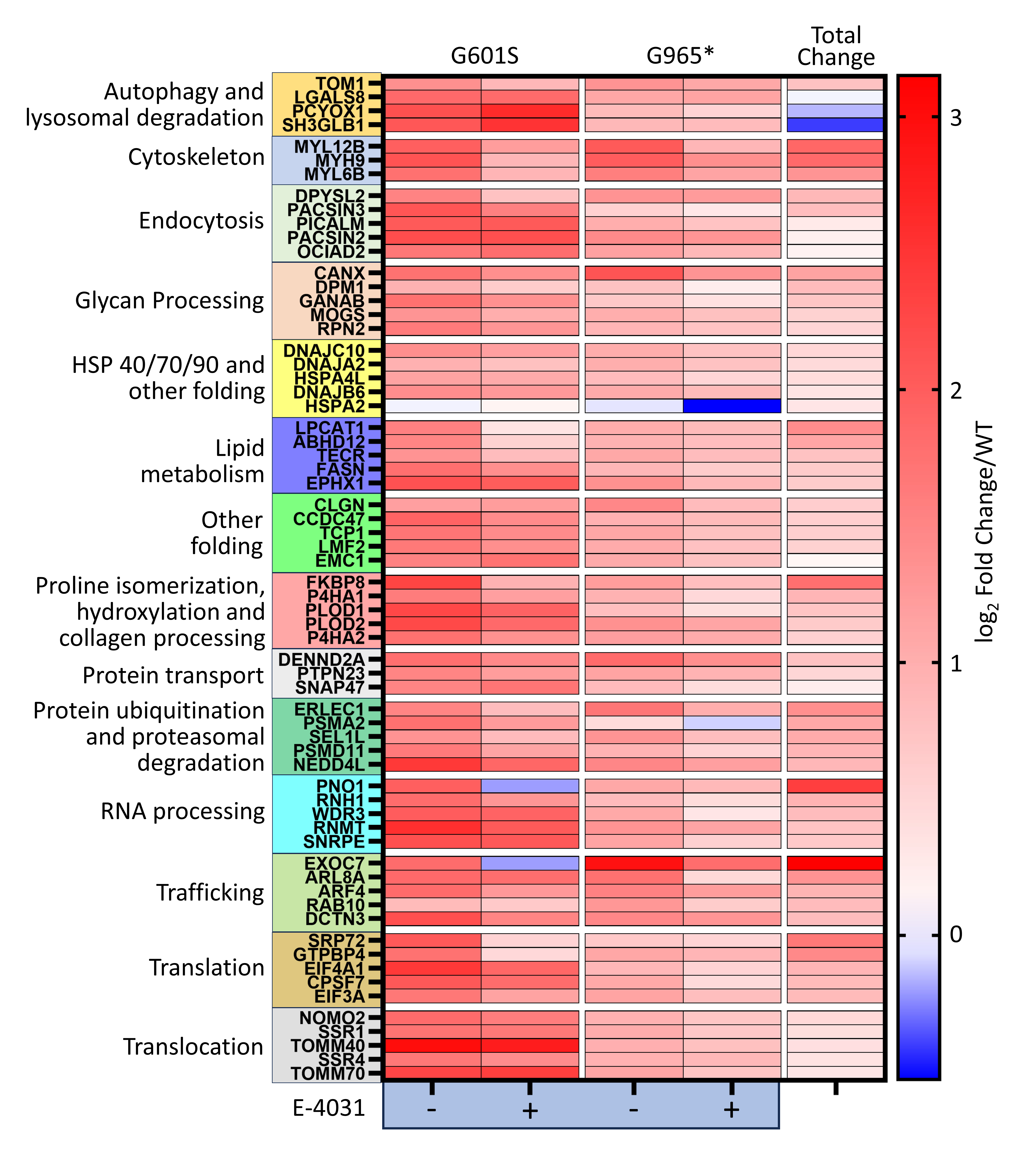
Results: We identified 572 protein interactions enriched in KV11.1-expressing cells. We used bioinformatic analysis to make a cellular model showcasing likely KV11.1 protein interactions localization from early biogenesis and transcription to plasma membrane expression (Figure 1). We revealed proteins responsible for protein folding, translation, proteasomal degradation, and others with most protein interactions significantly elevated in the genetic variants compared to wild-type (Figure 2). Upon 24-hour treatment with E-4031, some of these interactions were reduced towards wild-type and indicated potential molecular targets for therapeutic intervention (Figure 3).
Conclusions: We report the discovery of novel KV11.1 protein-protein interactions that could be therapeutically targeted to improve KV11.1 trafficking and treat Long QT Syndrome.
Methods: We used affinity-purification (e.g., coimmunoprecipitation) coupled with mass spectrometry to quantify protein interaction changes in human embryonic kidney cells expressing wild-type KV11.1 or two trafficking-deficient channel variants in the presence or absence of chemical chaperone E-4031. After co-immunoprecipitations and protein digestions, peptides were identified using multiplexed mass spectrometry.
Results: We identified 572 protein interactions enriched in KV11.1-expressing cells. We used bioinformatic analysis to make a cellular model showcasing likely KV11.1 protein interactions localization from early biogenesis and transcription to plasma membrane expression (Figure 1). We revealed proteins responsible for protein folding, translation, proteasomal degradation, and others with most protein interactions significantly elevated in the genetic variants compared to wild-type (Figure 2). Upon 24-hour treatment with E-4031, some of these interactions were reduced towards wild-type and indicated potential molecular targets for therapeutic intervention (Figure 3).
Conclusions: We report the discovery of novel KV11.1 protein-protein interactions that could be therapeutically targeted to improve KV11.1 trafficking and treat Long QT Syndrome.
More abstracts on this topic:
A-band titin-truncating variant promotes the development of arrhythmia-induced cardiomyopathy in a novel genetically-engineered porcine model
Lee Kwonjae, Del Rio Carlos, Mcnally Elizabeth, Pfenniger Anna, Bhatnagar Ashita, Glinton Kristofor, Burrell Amy, Ober Rebecca, Mcluckie Alicia, Bishop Brian, Rogers Christopher, Geist Gail
Acetylation of Electron Transfer Flavoprotein Alpha Is a Possible Regulatory Mechanism of Fatty Acid Oxidation in Diabetic HeartsTatekoshi Yuki, Yano Masaki, Hosoda Ryusuke, Saga Yukika, Kuno Atsushi