Final ID: MP2778
Effect of mavacamten treatment by duration of obstructive hypertrophic cardiomyopathy diagnosis: Results from the EXPLORER cohort of MAVA-Long-Term Extension study
Interim analyses from MAVA-LTE (NCT03723655) demonstrated that mavacamten is efficacious and well tolerated in patients with obstructive hypertrophic cardiomyopathy (oHCM). The relationship between the duration of oHCM diagnosis at mavacamten initiation and the effects of mavacamten are unknown.
Research Questions
To assess the effects of mavacamten in patients from the EXPLORER cohort of MAVA-LTE by duration of oHCM diagnosis.
Methods
All patients in MAVA-LTE received mavacamten. The effects of mavacamten on echocardiographic parameters and disease biomarker levels by duration of oHCM diagnosis were assessed (data cut-off: April 5, 2024).
Results
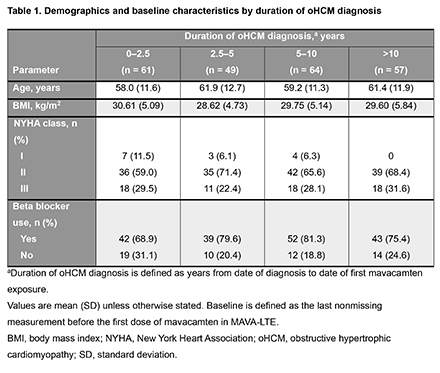
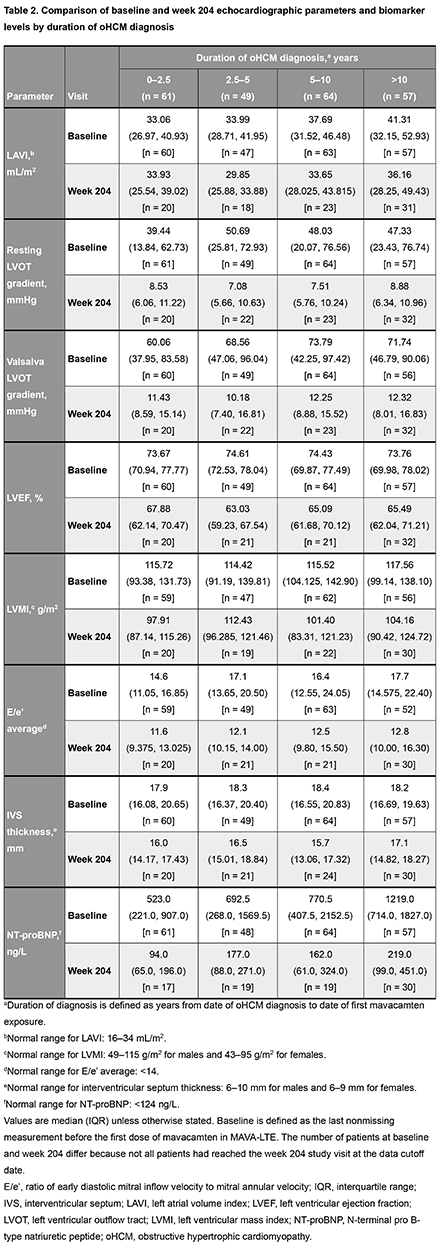
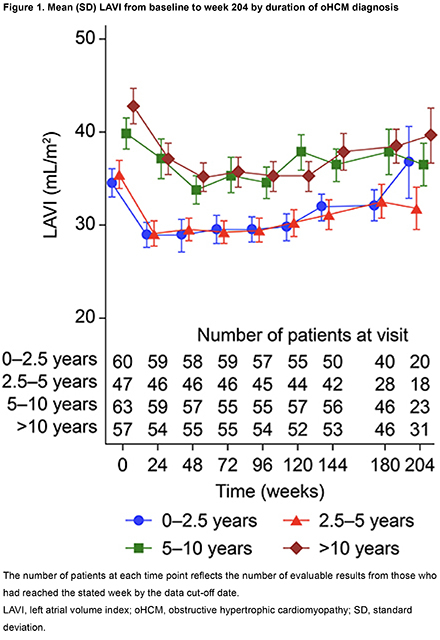
In total, 231 patients were categorized into 4 subgroups based on duration of oHCM diagnosis at mavacamten initiation: 0–2.5 years (n=61), 2.5–5 years (n=49), 5–10 years (n=64) and >10 years (n=57) (Table 1). At MAVA-LTE baseline, patients in the 0–2.5 years group had lower resting and Valsalva left ventricular outflow tract (LVOT) gradients, left atrial volume index (LAVI), interventricular septum (IVS) thickness, ratio of early diastolic mitral inflow velocity to mitral annular velocity (E/e’), and N-terminal pro B-type natriuretic peptide (NT-proBNP) levels than patients in other subgroups, while a higher proportion were New York Heart Association Class I (Table 1 and 2). By week 204, improvements in LAVI (Figure 1) were observed in all groups with abnormal baseline values (Table 2). For resting and Valsalva LVOT gradients, E/e’, LVMI, and NT-proBNP levels, values improved from baseline in all subgroups by week 204 (Table 2). IVS thickness improved from baseline in all subgroups by week 204 but remained elevated. Exposure adjusted incidence rates for serious treatment emergent adverse events per 100 patient-years of exposure were generally similar across subgroups (0–2.5 yrs: 9.30; 2.5–5 yrs: 7.04; 5–10 yrs: 12.33; >10 yrs: 11.53).
Conclusion
Patients in MAVA-LTE who had a shorter duration from diagnosis of oHCM to mavacamten initiation had lower values for key echocardiographic parameters and disease biomarker levels at baseline than those with a longer duration. Mavacamten treatment trended towards improvement in echocardiographic parameters and disease biomarker levels regardless of the duration of oHCM diagnosis.
- Owens, Anjali ( University of Pennsylvania , Philadelphia , Pennsylvania , United States )
- Stendahl, John ( Yale School of Medicine , New Haven , Connecticut , United States )
- Balaratnam, Ganesh ( Bristol Myers Squibb , Princeton , New Jersey , United States )
- Weerackoon, Nadisha ( Bristol Myers Squibb , Princeton , New Jersey , United States )
- Suryawanshi, Bharat ( Bristol Myers Squibb , Princeton , New Jersey , United States )
- Hegde, Sheila ( Brigham and Women’s Hospital , Boston , Massachusetts , United States )
- Olivotto, Iacopo ( Meyer Children’s Hospital, IRCCS , Florence , Italy )
- Oreziak, Artur ( National Institute of Cardiology , Warsaw , Poland )
- Barriales-villa, Roberto ( Complexo Hospitalario UniversitarioA Coruña, INIBIC, CIBERCV (ISCIII) , A Coruna , Spain )
- Abraham, Theodore ( University of California at San Francisco , San Francisco , California , United States )
- Wong, Timothy ( University of Pittsburgh Medical Center , Pittsburgh , Pennsylvania , United States )
- Fermin, David ( Corewell Health , Grand Rapids , Michigan , United States )
- Choudhury, Lubna ( Northwestern University, Feinberg School of Medicine , Chicago , Illinois , United States )
- Charron, Philippe ( Sorbonne Université, AP-HP, IHU-ICAN, INSERM 1166, Hôpital Universitaire Pitié-Salpêtrière , Paris , France )
- Seidler, Tim ( Kerckhoff Clinic , Bad Nauheim , Germany )
Meeting Info:
Session Info:
Innovation & Precision Medicine in Hypertrophic Cardiomyopathy
Monday, 11/10/2025 , 10:45AM - 11:35AM
Moderated Digital Poster Session
More abstracts on this topic:
Croon Philip, Aminorroaya Arya, Pedroso Aline, Dhingra Lovedeep, Khera Rohan
A Hypertrophic Cardiomyopathy Polygenic Score Modifies Penetrance of Pathogenic Hypertrophic and Dilated Cardiomyopathy Variants in Opposite DirectionsAbramowitz Sarah, Hoffman-andrews Lily, Depaolo John, Judy Renae, Owens Anjali, Damrauer Scott, Levin Michael
More abstracts from these authors:
Owens Anjali, Chen Yu Mao, Wang Andrew, Barriales-villa Roberto, Desai Milind, Garcia-pavia Pablo, Hagege Albert, Olivotto Iacopo, Wojakowski Wojtek, Afsari Sonia, Balaratnam Ganesh
Efficacy and Safety of Aficamten in Patients Guideline-Eligible for Septal Reduction Therapy in the FOREST-HCM TrialMasri Ahmad, Naidu Srihari, Nassif Michael, Olivotto Iacopo, Oreziak Artur, Owens Anjali, Wever-pinzon Omar, Tower Rader Albree, Heitner Stephen, Kupfer Stuart, Malik Fady, Choudhury Lubna, Melloni Chiara, Meng Lixin, Wei Jenny, Saberi Sara, Garcia-pavia Pablo, Abraham Theodore, Barriales-villa Roberto, Bilen Ozlem, Elliott Perry, Hagege Albert, Nagueh Sherif