Final ID: MDP894
Efficacy and Safety of Aficamten in Patients Guideline-Eligible for Septal Reduction Therapy in the FOREST-HCM Trial
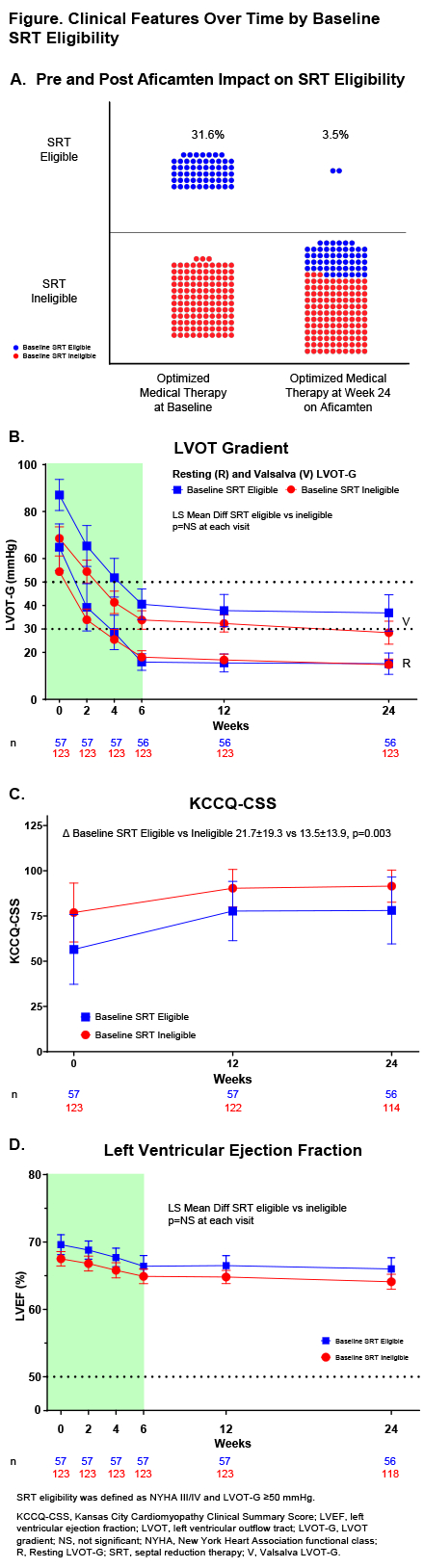
Methods. Pts completing an aficamten parent study were offered participation in FOREST-HCM. Aficamten was initiated in all participants at 5 mg and escalated (10, 15, and 20 mg) at investigators’ discretion with protocol guidance according to left ventricular ejection fraction (LVEF) and left ventricular outflow obstruction (LVOT) criteria. SRT eligibility was defined per guidelines as both New York Heart Association (NYHA) class III/IV and any peak LVOT-G ≥50 mmHg.
Results. Of 180 oHCM pts (mean age 60.8±13.1 years, 54% male) enrolled in FOREST-HCM from May 28, 2021 to March 15, 2024 with ≥24 weeks of follow-up, 57 (31.6%) were SRT-eligible at baseline. By week 24, only 2 (3.5%) pts remained SRT eligible (32 [56.1%] met neither criteria, 20 [35.1%] had LVOT-G ≥50 mmHg but were NYHA class I/II, and 2 (3.5%) had NYHA III/IV but LVOT-G <50 mmHg). NYHA improved ≥1 class in 52 (92.9%) SRT-eligible pts and in 80 (67.8%) pts of the remaining cohort. Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS) improved more in SRT-eligible pts than remaining cohort (21.7±19.3 vs 13.5±13.9; p=0.003) {Figure}. Proportional reductions from baseline in NT-proBNP and high-sensitivity troponin were substantial in both groups. A modest reduction in LVEF occurred in both groups (-3.6% ±6.4 vs -3.4% ±6.2 in the SRT-eligible and remaining cohort respectively; between group p=0.34) {Figure}.
Conclusions. A third of pts enrolled in FOREST-HCM were guideline SRT-eligible at baseline despite optimized standard of care therapy, with almost all pts no longer meeting SRT-eligibility criteria by 24 weeks with aficamten treatment. Aficamten may provide an effective alternative to SRT in some patients with oHCM.
- Masri, Ahmad ( OHSU , Portland , Oregon , United States )
- Naidu, Srihari ( Westchester Medical Center, New York Medical College , Valhalla , New York , United States )
- Nassif, Michael ( Saint Luke’s Mid America Heart Institute , Kansas City , Missouri , United States )
- Olivotto, Iacopo ( Meyer Children’s Hospital, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) , Florence , Italy )
- Oreziak, Artur ( National Institute of Cardiology , Warsaw , Poland )
- Owens, Anjali ( UNIVERSITY OF PENN , Walliford , Pennsylvania , United States )
- Wever-pinzon, Omar ( University of Utah , Salt Lake City , Utah , United States )
- Tower Rader, Albree ( Massachusetts General Hospital , Boston , Massachusetts , United States )
- Heitner, Stephen ( Cytokinetics Inc. , Portland , Oregon , United States )
- Kupfer, Stuart ( Cytokinetics , South San Francisco , California , United States )
- Malik, Fady ( CYTOKINETICS INC. , S San Fran , California , United States )
- Choudhury, Lubna ( NORTHWESTERN UNIVERSITY , Chicago , Illinois , United States )
- Melloni, Chiara ( Cytokinetics , South San Francisco , California , United States )
- Meng, Lixin ( CYTOKINETICS, INC. , South San Francisco , California , United States )
- Wei, Jenny ( Cytokinetics Inc. , Portland , Oregon , United States )
- Saberi, Sara ( University of Michigan , Ann Arbor , Michigan , United States )
- Garcia-pavia, Pablo ( Hospital Universitario Puerta de Hierro de Majadahonda, IDIPHISA, CIBERCV, and Centro Nacional de Investigaciones Cardiovasculares (CNIC) , Madrid , Spain )
- Abraham, Theodore ( Univ of California at San Francisco , San Francisco , California , United States )
- Barriales-villa, Roberto ( Complexo Hospitalario Universitario , A Coruna , Spain )
- Bilen, Ozlem ( Emory University School of Medicine , Atlanta , Georgia , United States )
- Elliott, Perry ( Barts Heart Centre and University College London , London , United Kingdom )
- Hagege, Albert ( Assistance Publique Hôpitaux de Paris, Hôpital Européen Georges-Pompidou , Paris , France )
- Nagueh, Sherif ( Houston Methodist DeBakey Heart and Vascular Center , Houston , Texas , United States )
Meeting Info:
Session Info:
More abstracts on this topic:
Abramowitz Sarah, Hoffman-andrews Lily, Depaolo John, Judy Renae, Owens Anjali, Damrauer Scott, Levin Michael
A Case of Hypertrophic Cardimyopathy: Digenic Variants of Uncertain Significance Mutations in MHY7 and RYR2 GenesDurukan Selina, Uzunoglu Ekin, Farahmandsadr Maryam, Soffer Daniel
More abstracts from these authors:
Hegde Sheila, Oreziak Artur, Owens Anjali, Tower Rader Albree, Heitner Stephen, Jacoby Daniel, Kupfer Stuart, Liu Xueli, Malik Fady, Melloni Chiara, Simkins Tyrell, Pabon Maria, Wei Jenny, Solomon Scott, Saberi Sara, Masri Ahmad, Nassif Michael, Abraham Theodore, Barriales-villa Roberto, Cooper Robert, Elliott Perry, Maron Martin
Long-term Impact of Aficamten on Patient-Reported Outcome Measures in Obstructive Hypertrophic Cardiomyopathy: Results From FOREST-HCMWeiner Shepard, Oreziak Artur, Saberi Sara, Solomon Scott, Spertus John, Tower Rader Albree, Butzner Michael, Heitner Stephen, Jacoby Daniel, Kupfer Stuart, Liu Xueli, Liang Lusha, Malik Fady, Melloni Chiara, Simkins Tyrell, Wei Jenny, Nassif Michael, Owens Anjali, Masri Ahmad, Abraham Theodore, Barriales-villa Roberto, Cooper Robert, Elliott Perry, De Feria Alejandro, Maron Martin