Final ID: MDP1371
Integrated safety and tolerability of mavacamten treatment over 5 years in patients with obstructive hypertrophic cardiomyopathy
Aim: To present integrated safety data of patients who received mavacamten treatment for obstructive HCM across 5 studies.
Methods: The pooled population included all patients who received ≥1 dose of mavacamten in PIONEER-HCM, PIONEER-Open Label Extension (data cut-off: May 31, 2022), EXPLORER-HCM, MAVA-Long-Term Extension (EXPLORER cohort; data cut-off: August 31, 2023) and VALOR-HCM including long-term extension (data cut-off: November 11, 2022).
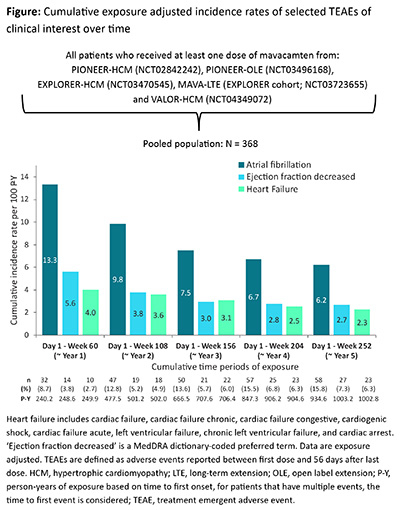
Results: The population consisted of 368 patients (mean [SD] age: 59.4 [12.2] years; male: 57.1%; White: 90.8%), including 350 patients who continued onto an extension study after completing a parent study. The majority of patients (57.6%) had a cumulative time on study of ≥3 years. Forty (10.9%) patients permanently discontinued treatment (13 due to AEs). In total, 20.7% of patients had a treatment emergent AE (TEAE) of Grade ≥3 and 14.7% had a TEAE leading to drug interruption. Overall, 4.3% of patients had drug-related serious TEAEs and 1.6% had a TEAE leading to death. The cumulative exposure adjusted incidence rates (EAIR) of all TEAEs, including AEs of clinical interest (atrial fibrillation [AF], ejection fraction [EF] decreased and heart failure [HF]), decreased as exposure increased over time, from 347.1/100 patient-years (P-Y) at Week 60 (~year 1) to 271.8/100 P-Y at Week 252 (~year 5). The EAIR of AF decreased over time from 13.3/100 P-Y at Week 60 (~year 1) to 6.2/100 P-Y at Week 252 (~year 5) (Figure). The EAIR for EF decreased and HF also decreased over time (Figure).
Conclusions: This integrated analysis of all patients with obstructive HCM treated with mavacamten across the 5 clinical studies over 5 years demonstrated that mavacamten treatment is generally well tolerated and that the EAIRs of selected TEAEs of clinical interest (AF, EF decreased and HF) decreased as exposure increased over time.
- Owens, Anjali ( University of Pennsylvania , Philadelphia , Pennsylvania , United States )
- Chen, Yu Mao ( Bristol Myers Squibb , Princeton , New Jersey , United States )
- Wang, Andrew ( Duke University Hospital , Durham , North Carolina , United States )
- Barriales-villa, Roberto ( Complexo Hospitalario Universitario , A Coruna , Spain )
- Desai, Milind ( Cleveland Clinic , Cleveland , Ohio , United States )
- Garcia-pavia, Pablo ( Hospital Puerta de Hierro Majadahonda , Madrid , Spain )
- Hagege, Albert ( AP-HP, Hôpital Européen Georges Pompidou , Paris , France )
- Olivotto, Iacopo ( Meyer Children’s Hospital IRCCS , Florence , Italy )
- Wojakowski, Wojtek ( Medical University of Silesia , Katowice , Poland )
- Afsari, Sonia ( Bristol Myers Squibb , Princeton , New Jersey , United States )
- Balaratnam, Ganesh ( Bristol Myers Squibb , Princeton , New Jersey , United States )
Meeting Info:
Session Info:
Monday, 11/18/2024 , 11:10AM - 12:35PM
Moderated Digital Poster Session
More abstracts on this topic:
Sangha Veer, Aminorroaya Arya, Dhingra Lovedeep, Pedroso Aline, Oikonomou Evangelos, Khera Rohan
Advanced Diagnosis of Hypertrophic Cardiomyopathy with AI-ECG and Differences Based on Race and SubtypeLewontin Myra, Perry Allison, Amos Kaitlyn, Ayers Michael, Kaplan Emily, Bilchick Kenneth, Barber Anita, Bivona Derek, Kramer Christopher, Parrish Anna, Mcclean Karen, Thomas Matthew
More abstracts from these authors:
Owens Anjali, Desai Milind
Effect of mavacamten treatment by duration of obstructive hypertrophic cardiomyopathy diagnosis: Results from the EXPLORER cohort of MAVA-Long-Term Extension studyOwens Anjali, Stendahl John, Balaratnam Ganesh, Weerackoon Nadisha, Suryawanshi Bharat, Hegde Sheila, Olivotto Iacopo, Oreziak Artur, Barriales-villa Roberto, Abraham Theodore, Wong Timothy, Fermin David, Choudhury Lubna, Charron Philippe, Seidler Tim