Final ID: MP1829
R-propranolol Attenuates Interleukin-18 Mediated Sickle Cell Cardiomyopathy: A Novel Adrenergic-independent Mechanism Involving Lipid Synthesis.
Abstract Body (Do not enter title and authors here): Background/Hypothesis: We previously reported that interleukin-18 (IL-18) mediates sickle cell cardiomyopathy (SCC), characterized by diastolic dysfunction, heart failure, and ventricular arrhythmias (VT). β-blockers such as propranolol are cardioprotective and reduce inflammation, but whether this benefit is derived from adrenergic signaling is unclear. We hypothesized that R-propranolol (enantiomer with 100-fold less adrenergic activity) may attenuate SCC via an adrenergic-independent pathway. Moreover, given propranolol induces lipid membrane remodeling, we further hypothesized that r-propranolol induces lipid synthesis and reduces membrane permeability resulting in reduced cardiac inflammation.
Methods: Human coronary artery endothelial cells (HCAECs) and humanized Townes sickle (SCD) mice (and controls) were treated for 24 hours with vehicle, r-propranolol, a known SREBP1 agonist (T0901317), and a known SREBP1 inhibitor (fatostatin). SREBP1 activity, subcellular localization, and IL-18 activation were assessed via immunofluorescence, western blotting, and RT-qPCR. Cardiac structure and function, electrophysiology, and histopathology were evaluated from in vivo and ex vivo testing to determine the therapeutic effects of R-propranolol (10uM, 7dd, IP).
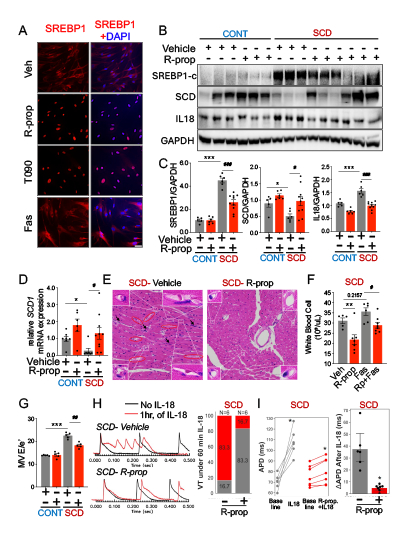
Results: In contrast to fatostatin, r-propranolol and T0901317 activated SREBP1 in HCAECs (Fig. 1A). Hearts from sickle mice exhibited increased IL-18 levels and reduced SREBP1 activity (reduced cleaved SREBP1 levels, increased stearoyl-CoA desaturase 1 or SCD1 levels- a known target for SREBP1) compared to control mice. Compared to vehicle, r-propranolol enhanced SREBP1 activity, reduced IL-18 levels, and cardiac inflammation (Figs. 1B-F) in both control and sickle mice which was attenuated by the concomitant use of fatostatin (data not shown). R-propranolol further improved diastolic dysfunction seen on echocardiography in vivo (Fig. 1G) and attenuated IL–18–induced action potential duration (APD) prolongation and VT ex vivo in sickle mice (Fig. 1H-I).
Conclusion: R-propranolol attenuates SCC via SREBP1 induction, resulting in reduced IL-18 activation and cardiac inflammation. These data mechanistically link SREBP1-mediated lipid synthesis to cardioprotection and support r-propranolol as a novel therapeutic for SCC with reduced adrenergic effects.
Methods: Human coronary artery endothelial cells (HCAECs) and humanized Townes sickle (SCD) mice (and controls) were treated for 24 hours with vehicle, r-propranolol, a known SREBP1 agonist (T0901317), and a known SREBP1 inhibitor (fatostatin). SREBP1 activity, subcellular localization, and IL-18 activation were assessed via immunofluorescence, western blotting, and RT-qPCR. Cardiac structure and function, electrophysiology, and histopathology were evaluated from in vivo and ex vivo testing to determine the therapeutic effects of R-propranolol (10uM, 7dd, IP).
Results: In contrast to fatostatin, r-propranolol and T0901317 activated SREBP1 in HCAECs (Fig. 1A). Hearts from sickle mice exhibited increased IL-18 levels and reduced SREBP1 activity (reduced cleaved SREBP1 levels, increased stearoyl-CoA desaturase 1 or SCD1 levels- a known target for SREBP1) compared to control mice. Compared to vehicle, r-propranolol enhanced SREBP1 activity, reduced IL-18 levels, and cardiac inflammation (Figs. 1B-F) in both control and sickle mice which was attenuated by the concomitant use of fatostatin (data not shown). R-propranolol further improved diastolic dysfunction seen on echocardiography in vivo (Fig. 1G) and attenuated IL–18–induced action potential duration (APD) prolongation and VT ex vivo in sickle mice (Fig. 1H-I).
Conclusion: R-propranolol attenuates SCC via SREBP1 induction, resulting in reduced IL-18 activation and cardiac inflammation. These data mechanistically link SREBP1-mediated lipid synthesis to cardioprotection and support r-propranolol as a novel therapeutic for SCC with reduced adrenergic effects.
More abstracts on this topic:
A Case of Hypertrophic Cardimyopathy: Digenic Variants of Uncertain Significance Mutations in MHY7 and RYR2 Genes
Durukan Selina, Uzunoglu Ekin, Farahmandsadr Maryam, Soffer Daniel
Perceived Discrimination from Healthcare Providers Among Individuals With and Without Sickle Cell Disease Who Have Suffered a StrokeTaylor Brittany, Conley Yvette