Final ID: Mo2111
VICTORION-INCEPTION: Consistent Low-density Lipoprotein Cholesterol Lowering With Inclisiran Following Acute Coronary Syndrome Independent of Index Lipid-lowering Therapy
Abstract Body (Do not enter title and authors here): Background: In the VICTORION-INCEPTION trial (NCT04873934), participants with recent acute coronary syndrome (ACS) randomized to receive inclisiran + usual care had greater low-density lipoprotein cholesterol (LDL-C) reductions and goal attainment than those randomized to usual care alone.
Research question: Given the variability in utilization of lipid-lowering therapy (LLT) at the time of index ACS in VICTORION-INCEPTION, we sought to assess if LLT utilization at time of index ACS event impacted LDL-C goal attainment and LDL-C lowering with inclisiran.
Methods: Eligible participants were screened within 5 weeks of discharge from hospitalization for ACS with LDL-C ≥70 mg/dL (or non–high-density lipoprotein cholesterol ≥100 mg/dL) and were receiving statin therapy or had statin intolerance. Participants were randomized 1:1 to inclisiran (inclisiran sodium 300 mg [284 mg inclisiran equivalent] on Days 0, 90, and 270) + usual care or to usual care alone. We present analyses of LDL-C <70 mg/dL and <55 mg/dL goal attainment and percentage change from baseline in LDL-C by index LLT utilization (captured in the case report form as LLT used prior to index ACS event).
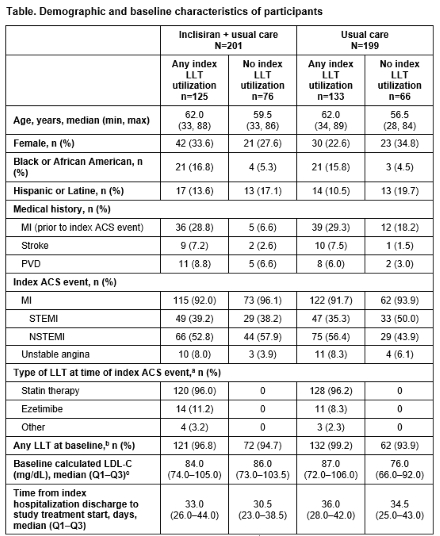
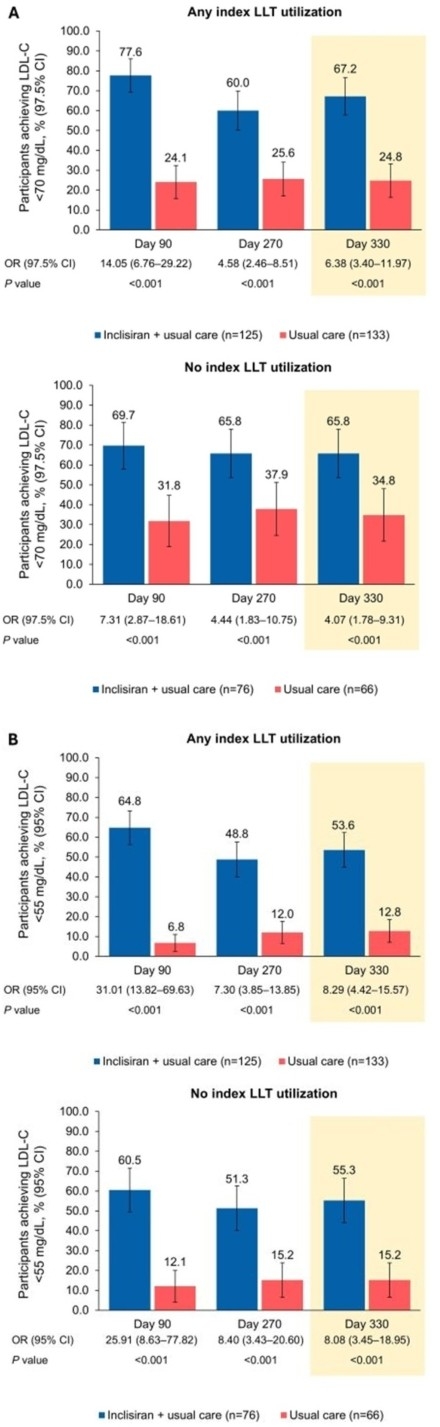
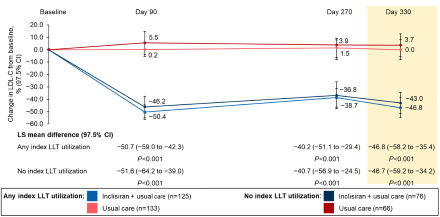
Results: Of 400 randomized participants, 64.5% were receiving index LLT (inclisiran + usual care: 62.2%; usual care: 66.8%) increasing to 96.8% of participants at the time of study treatment start (inclisiran + usual care: 96.0%; usual care: 97.5%; Table). Participants receiving index LLT had higher median age and higher rates of prior myocardial infarction. Irrespective of index LLT utilization, significantly more participants in the inclisiran + usual care arm, vs usual care, achieved LDLC <70 mg/dL from Day 90 through to Day 330 (LLT: ≥60.0% vs ≤25.6%; no LLT: ≥65.8% vs ≤37.9%; P<0.001 at all timepoints) and <55 mg/dL (LLT: ≥48.8% vs ≤12.8%; no LLT: ≥51.3% vs ≤15.2%; P<0.001 at all timepoints) (Figure 1). Similarly, LDLleast C least squares mean percentage change from baseline at Day 90 was significantly greater with inclisiran + usual care vs usual care (LLT: −50.4% vs 0.2%; no LLT: −46.2% vs 5.5%; both P<0.001) and was sustained to Day 330 (Figure 2).
Conclusion: Irrespective of index LLT utilization, in VICTORION-INCEPTION, treatment with inclisiran + usual care resulted in rapid and sustained LDL-C goal attainment and LDL-C lowering.
Research question: Given the variability in utilization of lipid-lowering therapy (LLT) at the time of index ACS in VICTORION-INCEPTION, we sought to assess if LLT utilization at time of index ACS event impacted LDL-C goal attainment and LDL-C lowering with inclisiran.
Methods: Eligible participants were screened within 5 weeks of discharge from hospitalization for ACS with LDL-C ≥70 mg/dL (or non–high-density lipoprotein cholesterol ≥100 mg/dL) and were receiving statin therapy or had statin intolerance. Participants were randomized 1:1 to inclisiran (inclisiran sodium 300 mg [284 mg inclisiran equivalent] on Days 0, 90, and 270) + usual care or to usual care alone. We present analyses of LDL-C <70 mg/dL and <55 mg/dL goal attainment and percentage change from baseline in LDL-C by index LLT utilization (captured in the case report form as LLT used prior to index ACS event).
Results: Of 400 randomized participants, 64.5% were receiving index LLT (inclisiran + usual care: 62.2%; usual care: 66.8%) increasing to 96.8% of participants at the time of study treatment start (inclisiran + usual care: 96.0%; usual care: 97.5%; Table). Participants receiving index LLT had higher median age and higher rates of prior myocardial infarction. Irrespective of index LLT utilization, significantly more participants in the inclisiran + usual care arm, vs usual care, achieved LDLC <70 mg/dL from Day 90 through to Day 330 (LLT: ≥60.0% vs ≤25.6%; no LLT: ≥65.8% vs ≤37.9%; P<0.001 at all timepoints) and <55 mg/dL (LLT: ≥48.8% vs ≤12.8%; no LLT: ≥51.3% vs ≤15.2%; P<0.001 at all timepoints) (Figure 1). Similarly, LDLleast C least squares mean percentage change from baseline at Day 90 was significantly greater with inclisiran + usual care vs usual care (LLT: −50.4% vs 0.2%; no LLT: −46.2% vs 5.5%; both P<0.001) and was sustained to Day 330 (Figure 2).
Conclusion: Irrespective of index LLT utilization, in VICTORION-INCEPTION, treatment with inclisiran + usual care resulted in rapid and sustained LDL-C goal attainment and LDL-C lowering.
More abstracts on this topic:
Association Between Elevated Lipoprotein(a) and New-Onset Atrial Fibrillation: A Retrospective Analysis Using the TriNetX Research Network
Qadeer Abdul, Akbar Usman, Ahmed Faizan, Shabbir Muhammad Raffey, Aamir Muhammad, Fouad Michele, Khan Allahdad, Khawar Muneeb, Pathak Prutha, Hassan Furqan, Hotwani Priya, Khan Sardar Muhammad Imran, Shafique Nouman
ACS-Specific Gut Microbial and Metabolic Profiles Reveal Diagnostic and Recovery MarkersXu Jing, Fu Jingyuan, Dai Die, Yang Yanan, Yang Jingang, Gao Shanshan, Wu Chongming, He Jiumin, Chen Weihua, Yang Yue-jin