Final ID: MP1492
VICTORION-INCEPTION: Adherence and Goal Attainment Data Support the Addition of Inclisiran to Background Lipid-Lowering Therapy as a Lipid Management Strategy Post-Acute Coronary Syndrome
Abstract Body (Do not enter title and authors here): Background: Guidelines recommend adding nonstatin lipid-lowering therapies (LLT) for patients receiving maximally tolerated statins post-acute coronary syndrome (ACS), if low-density lipoprotein cholesterol (LDL-C) goals are not met. In VICTORION-INCEPTION (NCT04873934), significantly more participants (pts) with recent ACS met LDL-C goals at Day 330 with inclisiran (INC) + usual care (UC) vs UC (<70 mg/dL: 66.7% vs 28.1%; <55 mg/dL: 54.2% vs 13.6%; both P<0.001).
Research question: How does addition of INC to UC post-ACS impact LLT adherence, utilization, and LDL-C lowering vs UC alone?
Methods: Eligible pts (discharged from ACS hospitalization ≤5 weeks before screening; LDL-C ≥70 mg/dL [or non–high-density lipoprotein cholesterol ≥100 mg/dL]; receiving statins or with documented statin intolerance) were randomized 1:1 to INC sodium 300 mg (284 mg INC equivalent) on Days 0, 90, and 270 + UC, or to UC alone. Adherence was evaluated by study days with self-reported LLT use as part of UC in both arms (%), using the case report form (secondary endpoint), and by Medication Adherence Report Scale-5 (MARS-5; scored 0–25; exploratory). Changes in UC LLT (secondary endpoint) and LDL-C goal attainment according to statin use at Day 330 (post hoc) were also assessed.
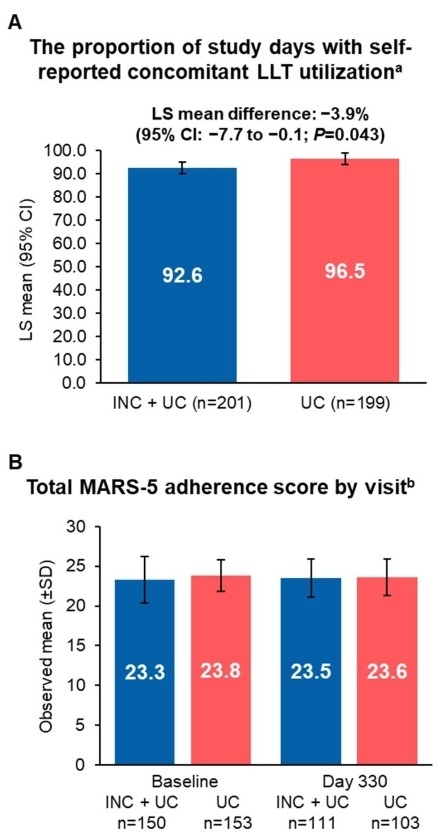
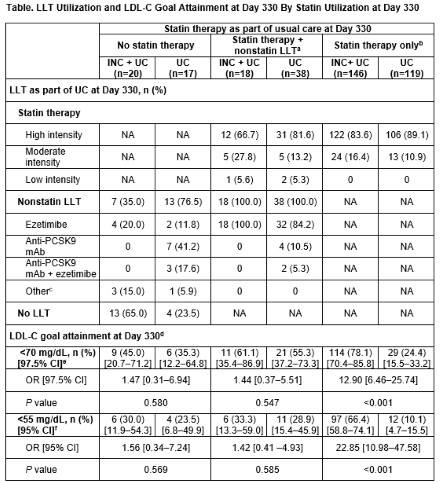
Results: In both arms, proportion of days with reported UC LLT use was high (INC + UC [n=201]: 92.6%, 95% CI: 90.0–95.3; UC [n=199]: 96.5%, 95% CI: 93.9–99.2; P=0.043); mean MARS-5 was >23 at baseline and Day 330 (Figure). Of pts with high intensity statin at baseline (INC + UC: 82.3%; UC: 81.7%), last postbaseline intensity for most was also high (INC + UC: 89.8%; UC: 95.0%). One pt in the INC + UC arm had nonstatin LLT added postbaseline, vs 20.3% (n=40) in the UC arm. Pts with INC + statin only at Day 330 had the highest proportion of LDL-C goal attainment and were significantly more likely to meet LDL-C goals vs pts with statin only (<70 mg/dL: 78.1% vs 24.4%, odds ratio [OR], 12.90, 97.5% CI, 6.46–25.74; <55 mg/dL: 66.4% vs 10.1%, OR, 22.85, 95% CI, 10.98–47.58; both P<0.001; Table). Pts receiving INC + no statin, or INC + statin + nonstatin LLT, were numerically more likely to meet LDL-C goals vs pts on UC at Day 330 (Table).
Conclusion: Adherence to LLT as part of UC was high with and without INC. Despite guideline recommendations, only 1 in 5 pts in the UC arm had LLT intensification with nonstatin LLT. These data support addition of INC to statins to improve lipid management post-ACS vs UC.
Research question: How does addition of INC to UC post-ACS impact LLT adherence, utilization, and LDL-C lowering vs UC alone?
Methods: Eligible pts (discharged from ACS hospitalization ≤5 weeks before screening; LDL-C ≥70 mg/dL [or non–high-density lipoprotein cholesterol ≥100 mg/dL]; receiving statins or with documented statin intolerance) were randomized 1:1 to INC sodium 300 mg (284 mg INC equivalent) on Days 0, 90, and 270 + UC, or to UC alone. Adherence was evaluated by study days with self-reported LLT use as part of UC in both arms (%), using the case report form (secondary endpoint), and by Medication Adherence Report Scale-5 (MARS-5; scored 0–25; exploratory). Changes in UC LLT (secondary endpoint) and LDL-C goal attainment according to statin use at Day 330 (post hoc) were also assessed.
Results: In both arms, proportion of days with reported UC LLT use was high (INC + UC [n=201]: 92.6%, 95% CI: 90.0–95.3; UC [n=199]: 96.5%, 95% CI: 93.9–99.2; P=0.043); mean MARS-5 was >23 at baseline and Day 330 (Figure). Of pts with high intensity statin at baseline (INC + UC: 82.3%; UC: 81.7%), last postbaseline intensity for most was also high (INC + UC: 89.8%; UC: 95.0%). One pt in the INC + UC arm had nonstatin LLT added postbaseline, vs 20.3% (n=40) in the UC arm. Pts with INC + statin only at Day 330 had the highest proportion of LDL-C goal attainment and were significantly more likely to meet LDL-C goals vs pts with statin only (<70 mg/dL: 78.1% vs 24.4%, odds ratio [OR], 12.90, 97.5% CI, 6.46–25.74; <55 mg/dL: 66.4% vs 10.1%, OR, 22.85, 95% CI, 10.98–47.58; both P<0.001; Table). Pts receiving INC + no statin, or INC + statin + nonstatin LLT, were numerically more likely to meet LDL-C goals vs pts on UC at Day 330 (Table).
Conclusion: Adherence to LLT as part of UC was high with and without INC. Despite guideline recommendations, only 1 in 5 pts in the UC arm had LLT intensification with nonstatin LLT. These data support addition of INC to statins to improve lipid management post-ACS vs UC.
More abstracts on this topic:
Atherosclerotic Cardiovascular Disease Risk Management in a Primary Care Residency Clinic
Manalo Kathryn, Tieliwaerdi Xiarepati, Jackson Megan, Arrigo Alexis, Mascara Mariah, Maharjan Srijana, Gadani Mrudula
A Novel Role for Lipoprotein(a) in Potentiating Neutrophil Extracellular Trap FormationMouawad Sahar, Boffa Michael, Koschinsky Marlys