Final ID: MP1257
Performance of a cuffless photoplethysmography-based device for continuous monitoring of blood pressure
Abstract Body (Do not enter title and authors here): Introduction
Blood pressure (BP) is a complex physiological marker. Cuffless devices, often based on photoplethysmography (PPG), enable frequent or continuous BP monitoring without arterial occlusion, enhancing comfort and usability in both inpatient and outpatient settings. Various devices have received regulatory clearance, but they are not yet included in clinical guidelines due to evolving validation standards.
Aim
To evaluate accuracy of the Biobeat WP613 device for continuous BP monitoring using invasive intra-arterial pressure (IAP) monitoring in ICCU patients.
Methods
This prospective, single-center study enrolled adults undergoing cardiac surgery at Chaim Sheba Medical Center (June 2020–January 2023) who had radial artery catheters for standard BP monitoring. Severe shock patients were excluded. Demographics and Fitzpatrick skin tone were recorded. ClinicalTrials.gov NCT04071015.
Post-op, patients were simultaneously monitored with the standard IAP system and the Biobeat device. Continuous BP and HR were collected for up to five days. Data analyzed post hoc.
Tested device
The WP613 (Biobeat, Israel) is FDA-cleared, chest-worn, PPG-based device that uses multi-wavelength light and a sensor to monitor vital signs. It wirelessly transmits data to a cloud based algorithms to measure BP, cardiac output, stroke volume, and more. It also records a single-lead ECG and has a 5-day battery life.
Results
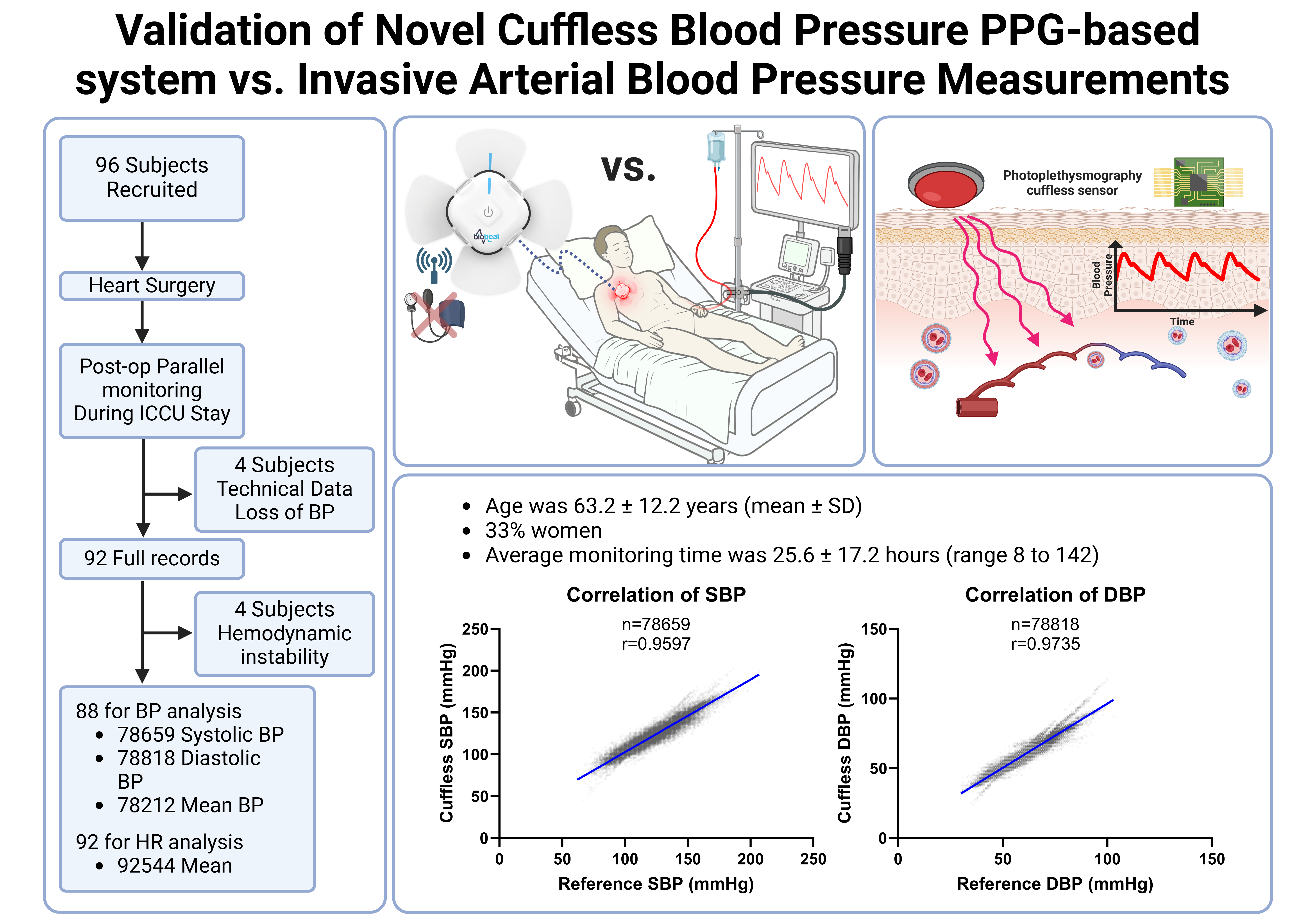
A total of 96 patients were enrolled, with 88 included in the final BP analysis after excluding 8 due to technical issues. Of those, 80 received inotropic support. The average age was 63.2±12.2 years, with 33% female. Monitoring lasted an average of 25.6±17.2 hours, yielding over 78000 readings for each BP parameter and over 92000 HR readings (Fig 1). Patients represented the full range of skin tones, classified using the Fitzpatrick scale I–VI.
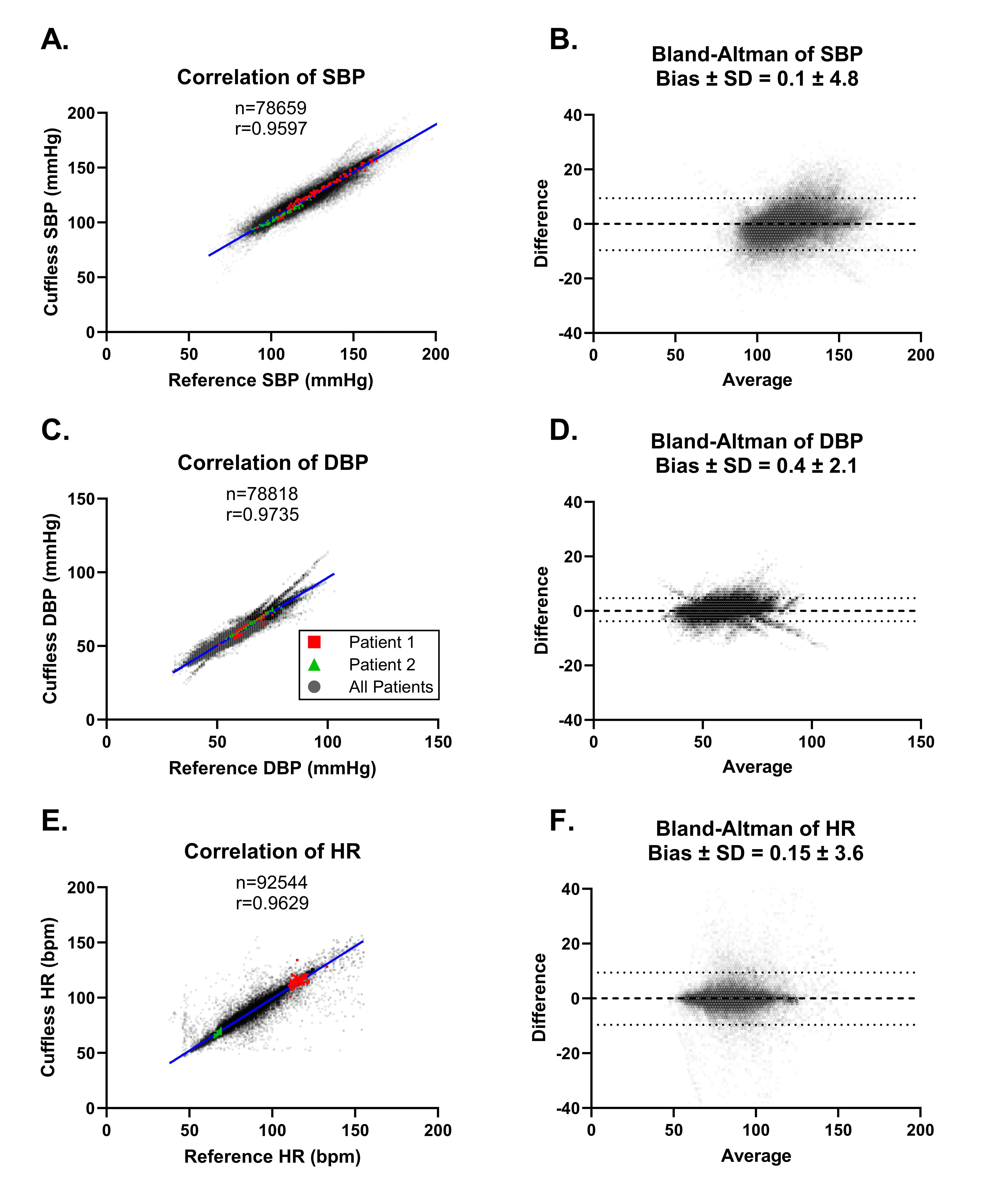
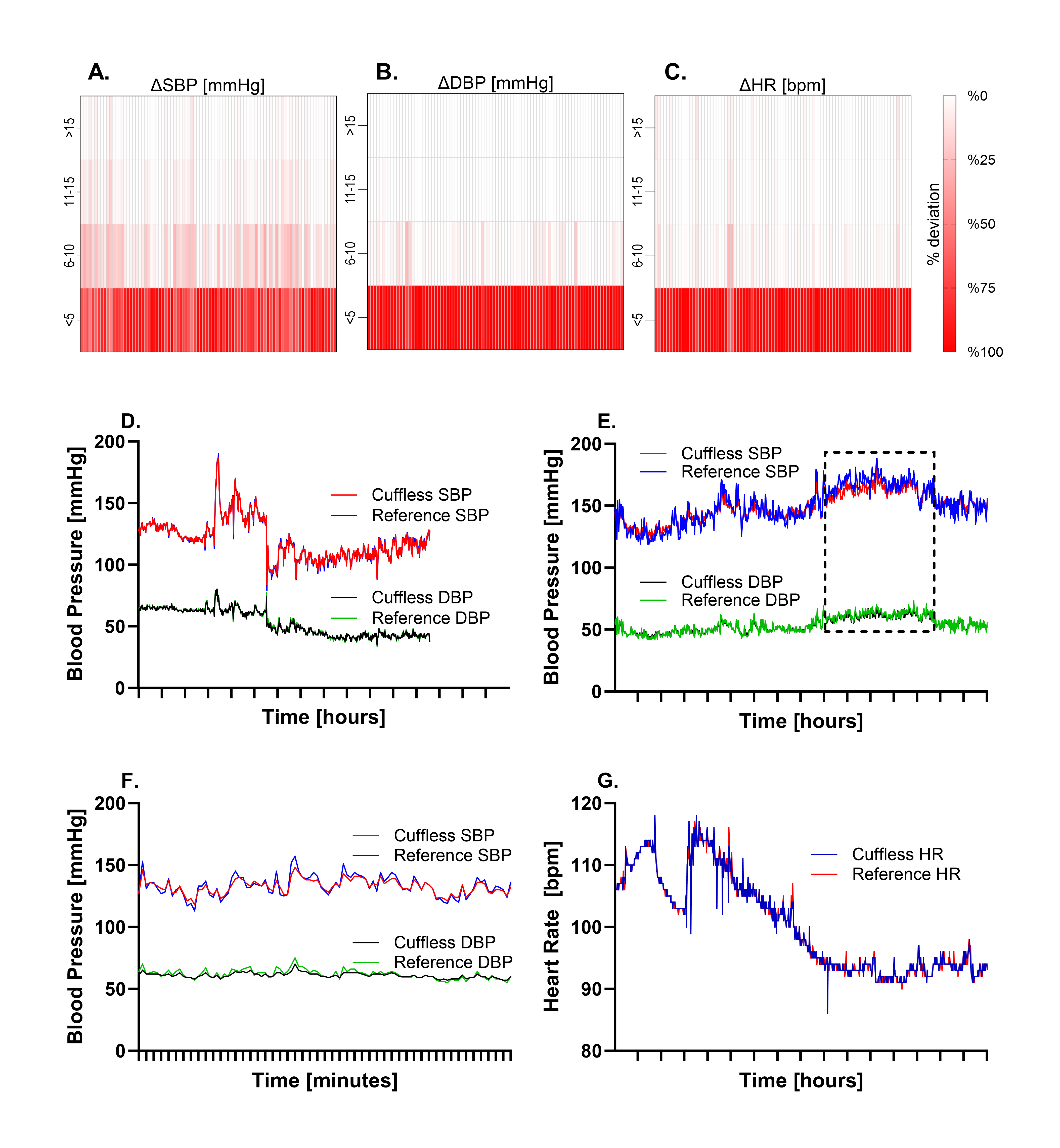
The Biobeat device showed excellent correlation with IAP monitoring: r=0.959 for SBP, 0.973 DBP, and 0.962 HR. Bland-Altman showed minimal bias (e.g., 0.1±4.8 for SBP) (Fig 2). Correlation remained strong across BP ranges and skin tones. Heatmaps and representative cases confirmed close tracking between Biobeat and IAP (Fig 3).
CONCLUSION
The tested cuffless device (Biobeat) offers a high level of accuracy and agreement for BP and HR compared to data obtained by IAP monitoring in post-cardiac surgery patients. Further studies are needed to validate these findings in various populations and clinical settings.
Blood pressure (BP) is a complex physiological marker. Cuffless devices, often based on photoplethysmography (PPG), enable frequent or continuous BP monitoring without arterial occlusion, enhancing comfort and usability in both inpatient and outpatient settings. Various devices have received regulatory clearance, but they are not yet included in clinical guidelines due to evolving validation standards.
Aim
To evaluate accuracy of the Biobeat WP613 device for continuous BP monitoring using invasive intra-arterial pressure (IAP) monitoring in ICCU patients.
Methods
This prospective, single-center study enrolled adults undergoing cardiac surgery at Chaim Sheba Medical Center (June 2020–January 2023) who had radial artery catheters for standard BP monitoring. Severe shock patients were excluded. Demographics and Fitzpatrick skin tone were recorded. ClinicalTrials.gov NCT04071015.
Post-op, patients were simultaneously monitored with the standard IAP system and the Biobeat device. Continuous BP and HR were collected for up to five days. Data analyzed post hoc.
Tested device
The WP613 (Biobeat, Israel) is FDA-cleared, chest-worn, PPG-based device that uses multi-wavelength light and a sensor to monitor vital signs. It wirelessly transmits data to a cloud based algorithms to measure BP, cardiac output, stroke volume, and more. It also records a single-lead ECG and has a 5-day battery life.
Results
A total of 96 patients were enrolled, with 88 included in the final BP analysis after excluding 8 due to technical issues. Of those, 80 received inotropic support. The average age was 63.2±12.2 years, with 33% female. Monitoring lasted an average of 25.6±17.2 hours, yielding over 78000 readings for each BP parameter and over 92000 HR readings (Fig 1). Patients represented the full range of skin tones, classified using the Fitzpatrick scale I–VI.
The Biobeat device showed excellent correlation with IAP monitoring: r=0.959 for SBP, 0.973 DBP, and 0.962 HR. Bland-Altman showed minimal bias (e.g., 0.1±4.8 for SBP) (Fig 2). Correlation remained strong across BP ranges and skin tones. Heatmaps and representative cases confirmed close tracking between Biobeat and IAP (Fig 3).
CONCLUSION
The tested cuffless device (Biobeat) offers a high level of accuracy and agreement for BP and HR compared to data obtained by IAP monitoring in post-cardiac surgery patients. Further studies are needed to validate these findings in various populations and clinical settings.
More abstracts on this topic:
A Non-Contacting Blood Flow Sensor for Assessing Pediatric Vascular Graft Patency
Chen Ruitong, Hudson Trevor, Wang Xuechun, Liang Jingjing, Nowlen Alanna, Nigam Vishal, Meng Ellis
A closed-loop system based on piezoelectric thin-film sensors and photothermal nanomaterials enables precise renal denervation for the treatment of hypertensionLiu Chengzhe, Zhou Liping, Yu Lilei