Final ID: MP1828
S100A9 Induces BRD4 (K292) Lactylation in Macrophages to Exacerbate Inflammaging in Diabetic Cardiomyopathy
Abstract Body (Do not enter title and authors here): Introduction: In age-related metabolic diseases such as diabetes, inflammaging exerts systemic pro-inflammatory responses and may disrupt interorgan communication and crosstalk. Previously, we have identified S100A9 (S100 calcium binding protein A9) as a critical mediator in the progression of diabetic cardiomyopathy (DCM). However, its association with cell senescence of DCM remains unclear.
Hypothesis: The activation of pro-inflammatory macrophages is typically driven by enhanced glycolysis. S100A9 significantly induces the inflammatory phenotypic switch of macrophage with lactate production. Hence, we hypothesized whether lactylation plays a critical regulatory role in this process.
Methods: We performed bioinformatics analyses on single-cell transcriptomics data from a heart failure model induced by diabetes and generating macrophage-specific S100A9 knockout mice. Lactylation modification proteomics was performed for identification of potential targets of S100A9. In BRD4(K292R) mutant cells , we conducted senescence-related functional assays.
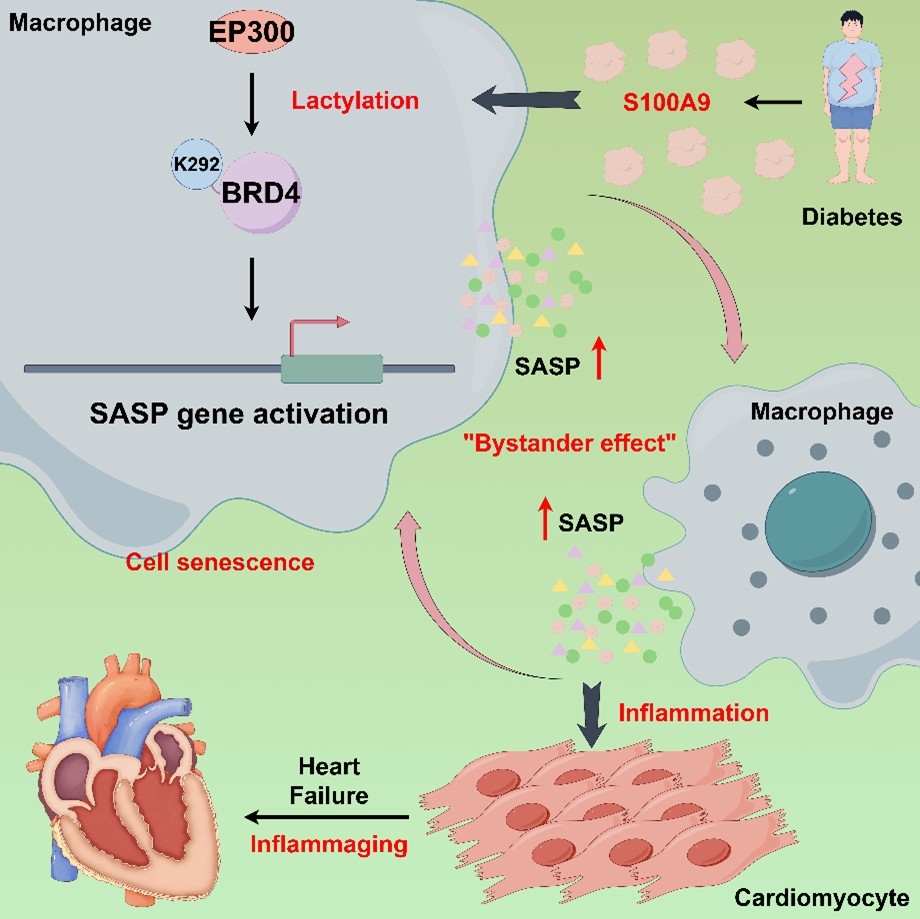
Results: We found that diabetes triggers the activation of circulating and tissue-resident CCR2+S100A9+macrophages, with serum S100A9 and lactate increased in Streptozotocin/ High-Fat Diet induced mice. By lactylation modification proteomics, we identified BRD4 (K292) as one of the significantly altered modification sites upon S100A9 induction in macrophages. We extracted the macrophages of Streptozotocin/ High-Fat Diet induced and S100A9 macrophage-specific knockout mice, and validated that S100A9 enhances BRD4(K292) lactylation with co-immunoprecipitation. Additionally, treatment with recombinant S100A9 protein increased the expression of the senescence-associated secretory phenotype (SASP). In vitro, mutating BRD4(K292) in macrophages reversed the cell senescence effect induced by S100A9.
Conclusion: Targeting the S100A9-induced BRD4 (K292) lactylation pathway in macrophages provides a novel therapeutic strategy for diabetic myocardial injury through suppressing inflammaging.
Hypothesis: The activation of pro-inflammatory macrophages is typically driven by enhanced glycolysis. S100A9 significantly induces the inflammatory phenotypic switch of macrophage with lactate production. Hence, we hypothesized whether lactylation plays a critical regulatory role in this process.
Methods: We performed bioinformatics analyses on single-cell transcriptomics data from a heart failure model induced by diabetes and generating macrophage-specific S100A9 knockout mice. Lactylation modification proteomics was performed for identification of potential targets of S100A9. In BRD4(K292R) mutant cells , we conducted senescence-related functional assays.
Results: We found that diabetes triggers the activation of circulating and tissue-resident CCR2+S100A9+macrophages, with serum S100A9 and lactate increased in Streptozotocin/ High-Fat Diet induced mice. By lactylation modification proteomics, we identified BRD4 (K292) as one of the significantly altered modification sites upon S100A9 induction in macrophages. We extracted the macrophages of Streptozotocin/ High-Fat Diet induced and S100A9 macrophage-specific knockout mice, and validated that S100A9 enhances BRD4(K292) lactylation with co-immunoprecipitation. Additionally, treatment with recombinant S100A9 protein increased the expression of the senescence-associated secretory phenotype (SASP). In vitro, mutating BRD4(K292) in macrophages reversed the cell senescence effect induced by S100A9.
Conclusion: Targeting the S100A9-induced BRD4 (K292) lactylation pathway in macrophages provides a novel therapeutic strategy for diabetic myocardial injury through suppressing inflammaging.
More abstracts on this topic:
Age and White Matter Injury due to Cerebral Small Vessel Disease are Synergistically Associated with Impaired Neurovascular Coupling.
Yang Sheng, Webb Alastair
Aficamten is safe and effective in oHCM with comorbidities obesity, hypertension, and diabetes: a SEQUOIA-HCM sub-studyLee Matthew, Malik Fady, Kupfer Stuart, Wohltman Amy, Coats Caroline, Abraham Theodore, Claggett Brian, Maron Martin, Miao Zi, Meder Benjamin, Olivotto Iacopo, Heitner Stephen, Jacoby Daniel