Final ID: MP1634
Allograft Outcomes of Adding Proprotein Convertase Subtilisin Kexin 9 Inhibitors or Ezetimibe to Statin Therapy in Heart Transplants Recipients at High Risk of Cardiac Allograft Progression: A Multicenter Target Trial Emulation
Abstract Body (Do not enter title and authors here): Introduction
Proprotein convertase subtilisin kexin 9 inhibitors (PCSK9i) and ezetimibe improve cardiovascular outcomes in patients with inadequate lipid control. Current literature describes improved LDL control and decreased cardiac allograft vasculopathy (CAV) progression in heart transplant (HT) recipients, particularly in patients taking combined PCSK9i and statin therapy. However, comparative data of additional PCSK9i vs ezetimibe to statin therapy is limited, particularly in multicenter settings.
Research Question
In HT recipients at high risk of CAV progression or allograft failure, will adding PCSK9i to statin therapy improve allograft outcomes compared to ezetimibe + statin therapy?
Methods
In this U.S.-based multicenter study (TriNetX dataset), adult HT recipients (≥18 years, 2010-2024) with known CAV, cardiovascular risk factors or LDL ≥ 70 mg/dl, non-HDL ≥ 100 despite statin therapy were identified. Two treatments were compared: initiation of PCSK9i + statin vs ezetimibe + statin. The start of follow-up (time zero) was defined as first prescription date of either combination. Those with familial hypercholesterolemia (FH) or concurrent PCSK9i + ezetimibe use were excluded. Propensity score matching (1:1) balanced the groups by comorbidities, CAV, and medications. Patients were followed at 3-year and 5-year intervals, excluding FH and concurrent PCSK9i + ezetimibe use. Primary outcome was allograft failure; secondary outcomes were new CAV, all-cause mortality, and LDL levels. Kaplan-Meier analysis and log-rank tests compared outcomes, continuous variables were compared using independent two-sample t-test, hazard ratios with 95% CI were calculated using Cox regression.
Results
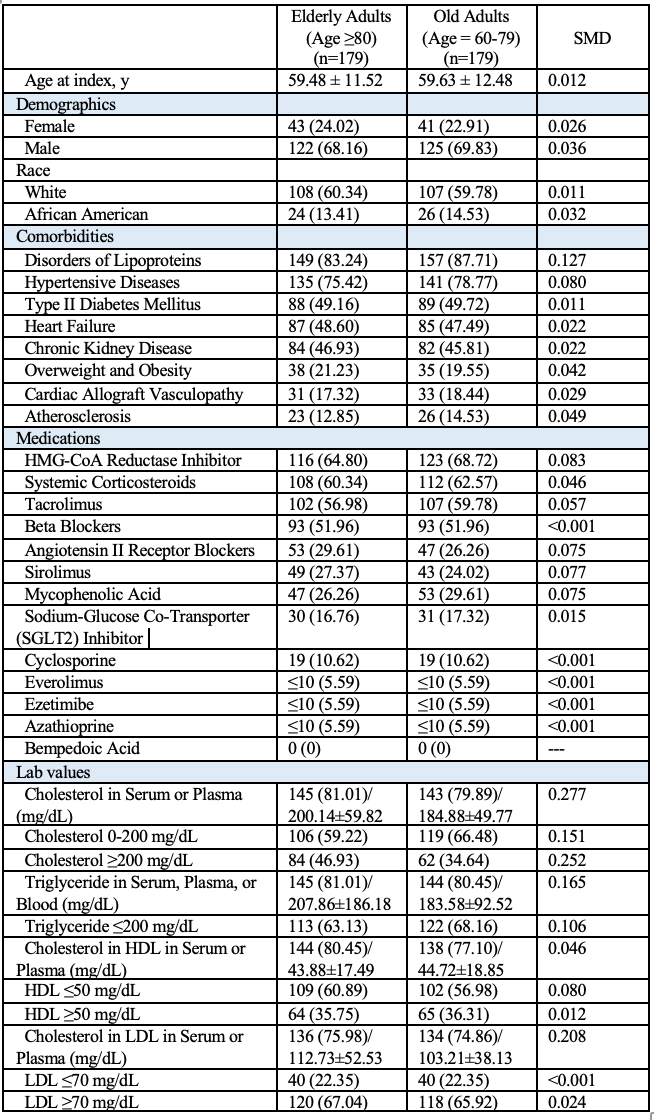
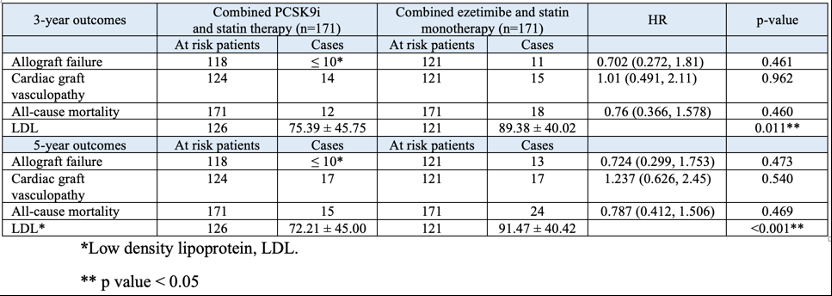
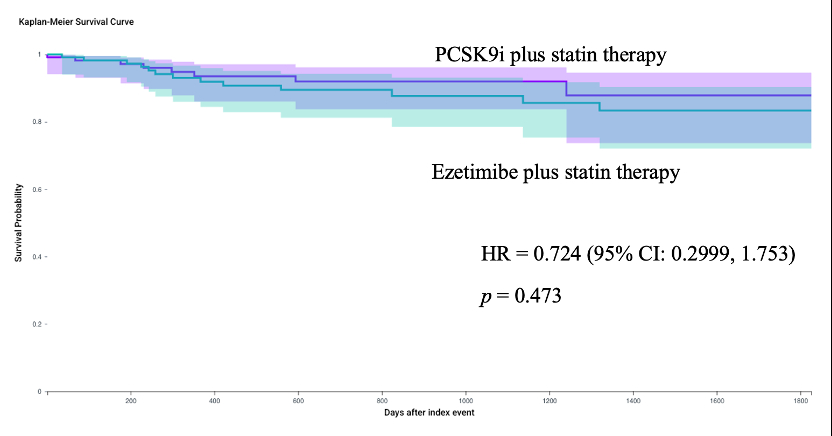
After matching into well-balanced groups (N=171 per group at 3-year and 5-year follow-up) at 3-years, outcomes did not differ significantly except for lower LDL levels in PCSK9i users (75.39 ± 45.75 vs. ezetimibe 89.38 ± 40.02 mg/dl, p = 0.011). At 5-years, no significant differences in outcomes, with the PCSK9i group maintaining lower LDL levels (72.21 ± 45.00 vs ezetimibe 91.47 ± 40.42, p <0.001).
Conclusions
In HT recepients at high risk of CAV progression or allograft failure, PCSK9i or ezetimibe added to statin therapy demonstrated comparable outcomes in allograft failure and new CAV diagnosis. PCSK9i users continued to show significantly lower LDL levels. While this supports the efficacy of ezetimibe and PCSK9i, prospective investigations are needed to review these results.
Proprotein convertase subtilisin kexin 9 inhibitors (PCSK9i) and ezetimibe improve cardiovascular outcomes in patients with inadequate lipid control. Current literature describes improved LDL control and decreased cardiac allograft vasculopathy (CAV) progression in heart transplant (HT) recipients, particularly in patients taking combined PCSK9i and statin therapy. However, comparative data of additional PCSK9i vs ezetimibe to statin therapy is limited, particularly in multicenter settings.
Research Question
In HT recipients at high risk of CAV progression or allograft failure, will adding PCSK9i to statin therapy improve allograft outcomes compared to ezetimibe + statin therapy?
Methods
In this U.S.-based multicenter study (TriNetX dataset), adult HT recipients (≥18 years, 2010-2024) with known CAV, cardiovascular risk factors or LDL ≥ 70 mg/dl, non-HDL ≥ 100 despite statin therapy were identified. Two treatments were compared: initiation of PCSK9i + statin vs ezetimibe + statin. The start of follow-up (time zero) was defined as first prescription date of either combination. Those with familial hypercholesterolemia (FH) or concurrent PCSK9i + ezetimibe use were excluded. Propensity score matching (1:1) balanced the groups by comorbidities, CAV, and medications. Patients were followed at 3-year and 5-year intervals, excluding FH and concurrent PCSK9i + ezetimibe use. Primary outcome was allograft failure; secondary outcomes were new CAV, all-cause mortality, and LDL levels. Kaplan-Meier analysis and log-rank tests compared outcomes, continuous variables were compared using independent two-sample t-test, hazard ratios with 95% CI were calculated using Cox regression.
Results
After matching into well-balanced groups (N=171 per group at 3-year and 5-year follow-up) at 3-years, outcomes did not differ significantly except for lower LDL levels in PCSK9i users (75.39 ± 45.75 vs. ezetimibe 89.38 ± 40.02 mg/dl, p = 0.011). At 5-years, no significant differences in outcomes, with the PCSK9i group maintaining lower LDL levels (72.21 ± 45.00 vs ezetimibe 91.47 ± 40.42, p <0.001).
Conclusions
In HT recepients at high risk of CAV progression or allograft failure, PCSK9i or ezetimibe added to statin therapy demonstrated comparable outcomes in allograft failure and new CAV diagnosis. PCSK9i users continued to show significantly lower LDL levels. While this supports the efficacy of ezetimibe and PCSK9i, prospective investigations are needed to review these results.
More abstracts on this topic:
A 10-year longitudinal cohort study of lipid variability, cognitive decline, and dementia in 9846 community-dwelling older adults
Zhou Zhen, Moran Chris, Murray Anne, Zoungas Sophia, Nelson Mark, Talic Stella, Wolfe Rory, Ryan Joanne
A Randomized Phase 2 Trial of Muvalaplin: An Oral Disrupter of the Assembly of Lipoprotein(a) ParticlesNicholls Stephen, Ni Wei, Rhodes Grace, Nissen Steven, Navar Ann Marie, Michael Laura, Krege John