Final ID: MDP858
Relationship Between CVH and Survival in the Acoramidis Treated Participants Within ATTRibute-CM
Hypothesis: CVH portends a higher risk of mortality in participants with ATTR-CM. Since acoramidis reduces CVH, it can improve the prognosis of participants with ATTR-CM.
Aim: To evaluate the relationship between CVH and survival in the acoramidis group within ATTRibute-CM.
Methods: In this post-hoc analysis, the relationship between those with or without CVH and survival was analyzed within the acoramidis treatment group using the Kaplan-Meier (KM) estimator method.
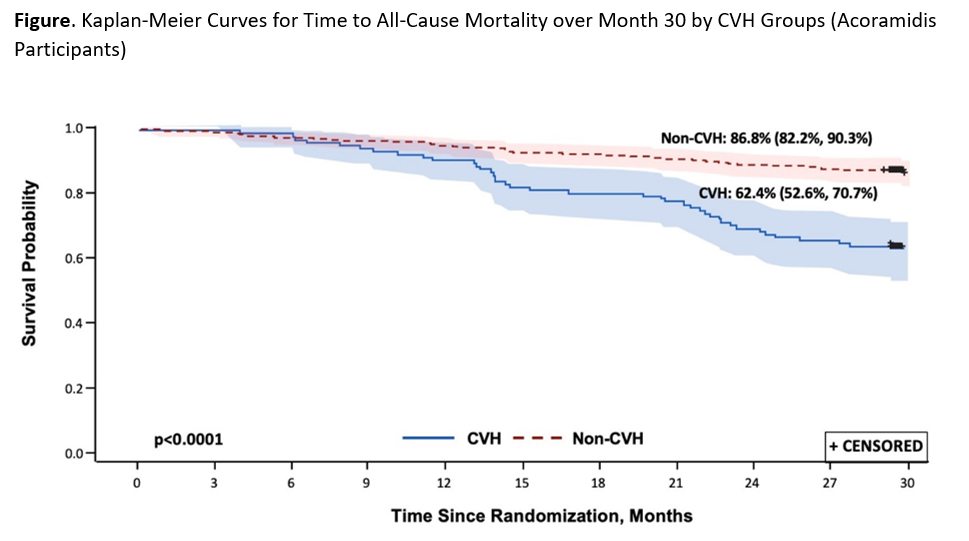
Results: Demographics and baseline disease characteristics were mostly comparable between acoramidis-treated participants with and without CVH, although participants with CVH had a higher baseline NT-proBNP and lower eGFR. At Month 30, in acoramidis-treated participants, those without any CVH (n=300) had a higher survival [86.8% (95% CI = 82.2, 90.3)] versus 62.4% (95% CI = 52.6, 70.7) in those who had any CVH (n=109); p<0.0001 from log-rank test (Figure).
Conclusion: In the ATTRibute-CM study, pts without any CVH receiving acoramidis have a higher survival rate. CVHs remain a powerful predictor of mortality. This reinforces the importance of an effective therapy that reduces CVH and in turn may improve survival in patients with ATTR-CM.
- Alexander, Kevin ( Stanford University , Palo Alto , California , United States )
- Whang, John ( BridgeBio Pharma , New York , New York , United States )
- Fox, Jonathan ( BridgeBio Pharma , New York , New York , United States )
- Du, Jing ( BridgeBio Pharma , New York , New York , United States )
- Cheng, Richard ( University of Washington , Seattle , Washington , United States )
- Davis, Margot ( UNIVERSITY OF BRITISH COLUMBIA , Vancouver , British Columbia , Canada )
- Ambardekar, Amrut ( University of Colorado , Aurora , Colorado , United States )
- Nativi-nicolau, Jose ( Mayo Clinic , Jacksonville , Florida , United States )
- Shah, Keyur ( VIRGINIA COMMONWEALTH UNIVERSITY , Richmond , Virginia , United States )
- Hanna, Mazen ( CLEVELAND CLINIC , Cleveland , Ohio , United States )
- Gibbs, Simon ( BridgeBio Pharma , New York , New York , United States )
- Tamby, Jean-francois ( BridgeBio Pharma , New York , New York , United States )
- Siddhanti, Suresh ( BridgeBio Pharma , New York , New York , United States )
Meeting Info:
Session Info:
Cardiac Amyloidosis 2024: Advances in Prognostication and Management
Sunday, 11/17/2024 , 11:10AM - 12:35PM
Moderated Digital Poster Session
More abstracts on this topic:
Jiang Chao, Dong Jianzeng, Cai Jun, Anderson Craig, Du Xin, Tang Yangyang, Han Rong, Song Yanna, Wang Chi, Lin Xiaolei, Yi Yang, Rodgers Anthony, Ma Changsheng
Acoramidis Improved Clinical Outcomes, Function, Quality of Life and NT-proBNP in Patients With Transthyretin Amyloid Cardiomyopathy Regardless of Atrial Fibrillation Status at BaselineSperry Brett, Tamby Jean-francois, Castano Adam, Fox Jonathan, Cheng Richard, Judge Daniel, Cappelli Francesco, Masri Ahmad, Grogan Martha, Mooney Deirdre, Akinboboye Olakunle, Drachman Brian, Nativi-nicolau Jose, Kobayashi Masatake, Chen Chris
More abstracts from these authors:
Davis Margot, Soman Prem, Kittleson Michelle, Berk John, Cao Xiaofan, Tamby Jean-francois, Castano Adam, Fox Jonathan, Shah Keyur, Grogan Martha, Griffin Jan, Sarswat Nitasha, Grodin Justin, Alexander Kevin, Judge Daniel, Gillmore Julian, Cappelli Francesco, Wright Richard
Acoramidis Reduces the Risk of All-Cause Mortality and Cardiovascular-Related Hospitalization Compared With Placebo in Participants With Transthyretin Amyloid Cardiomyopathy and Early-Stage Heart Failure Regardless of Atrial Fibrillation History: Insights From ATTRibute-CMWitteles Ronald, Mitter Sumeet, Gillmore Julian, Hanna Mazen, Berk John, Mitchell Joshua, Shah Keyur, Kobayashi Masatake, Xiong Kuangnan, Castano Adam, Tamby Jean-francois, Fox Jonathan