Final ID: Mo4072
Early Reported Events with the TriClip™ System for Transcatheter Tricuspid Valve Repair: Insights from FDA's MAUDE Database
Abstract Body (Do not enter title and authors here): Background: Tricuspid regurgitation (TR) worsens heart failure symptoms and perpetuates right ventricular failure (RVF). Given the limited efficacy of medicines and high risk of surgical mortality, percutaneous therapeutic options are gaining importance. The TRILUMINATE study reported an 86% reduction in TR severity and 4% mortality rate using Triclip G4 tricuspid transcatheter edge-to-edge repair (T-TEER) system with improvement in health status. Triclip subsequently gained FDA approval for TR on April 2, 2024.
Objective: To evaluate reported device and patient related adverse events during early experience with Triclip system for T-TEER.
Methods: The events reported for Triclip since it gained FDA approval were extracted from the FDA MAUDE database. Previously published reports, duplicates and events before FDA approval were excluded. Grades of TR at baseline and after T-TEER associated with single leaflet device attachment (SLDA) were compared using Wilcoxon rank sum test.
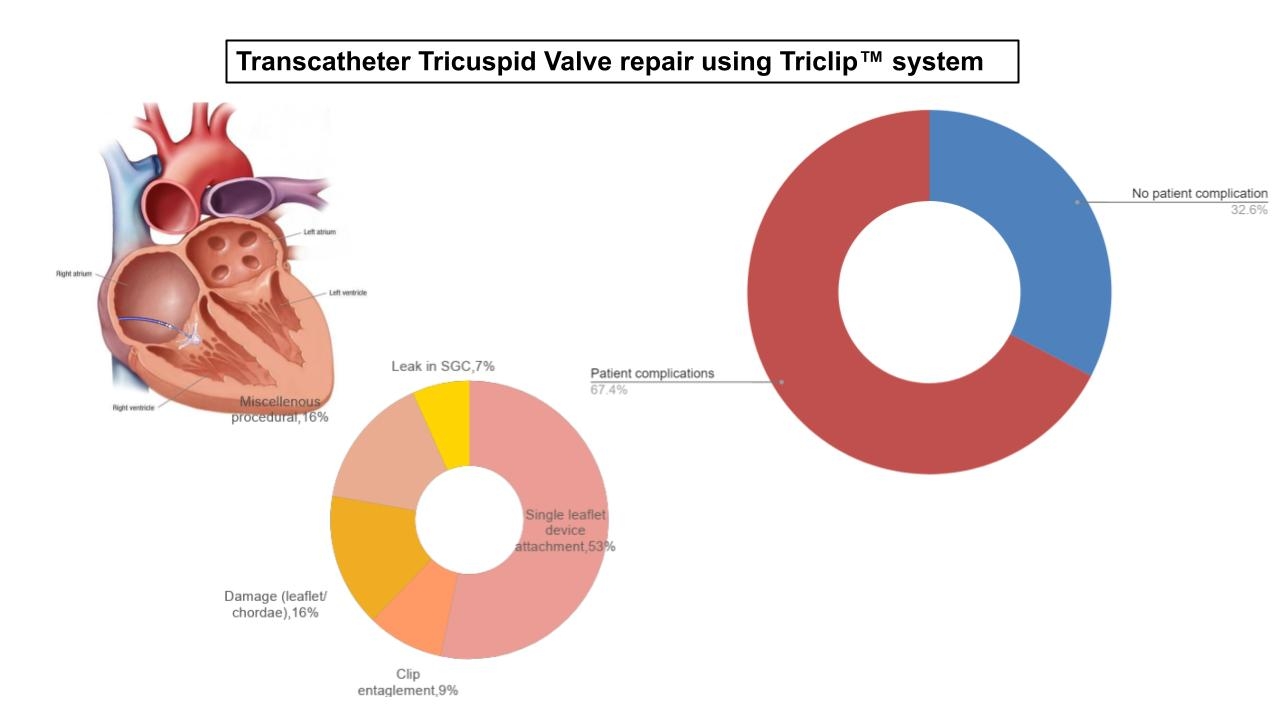
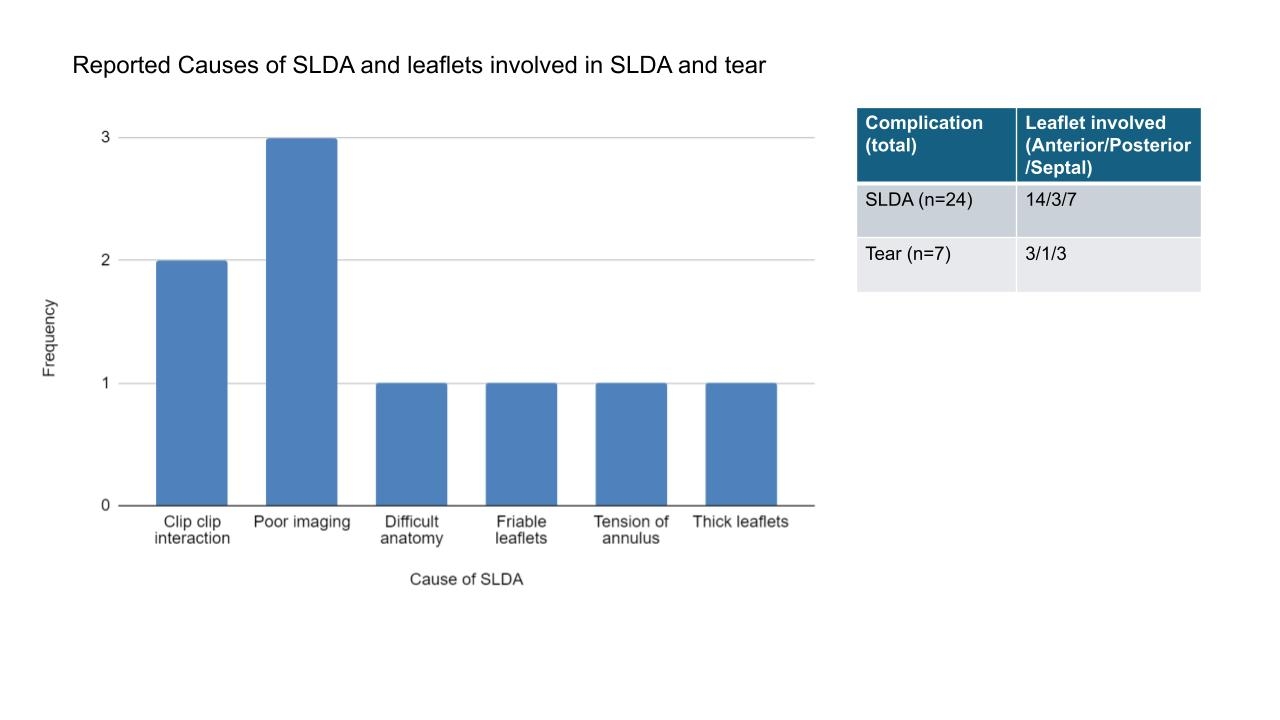
Results: After excluding 14 reports, 45 were included, dating from 04/02/24 to 05/31/24. Of these, 31 (67.4%) featured patient complications, with SLDA being the most frequent (n=24, 53%).(Figure-1) Cause of SLDA was reported in 8 reports.(Figure-2) SLDA led to regression of TR to pre-procedure levels in 10 patients and Polymorphic VT in one patient. Other patient issues included damage to leaflets (n=7, 15.6%) which necessitated surgery in one case and prompted consideration of the same in another. There were 4 reports of clip entrapment in the chordae. Device-related issues included 3 cases of leaks in the steerable guide catheter affecting its ability to hold the column, knotting on the lock line, difficulties with positioning the second clip above the valve, clip reopening beyond the expected 5°, clip opening while locked but staying closed post-deployment, delays in clip delivery, and challengers in guiding catheter positioning. No acute deaths were reported in the MAUDE database within 2 months of device approval.
Conclusion:
Our research findings summarize the reported adverse events during the early period following FDA approval of Triclip G4 T-TEER system. This provides valuable insights into common failure modes and complications, offering guidance on their optimal utilization. Multiple adverse events can be noted soon after approval of the Triclip, underscoring the importance of good initial training and proctoring.
Objective: To evaluate reported device and patient related adverse events during early experience with Triclip system for T-TEER.
Methods: The events reported for Triclip since it gained FDA approval were extracted from the FDA MAUDE database. Previously published reports, duplicates and events before FDA approval were excluded. Grades of TR at baseline and after T-TEER associated with single leaflet device attachment (SLDA) were compared using Wilcoxon rank sum test.
Results: After excluding 14 reports, 45 were included, dating from 04/02/24 to 05/31/24. Of these, 31 (67.4%) featured patient complications, with SLDA being the most frequent (n=24, 53%).(Figure-1) Cause of SLDA was reported in 8 reports.(Figure-2) SLDA led to regression of TR to pre-procedure levels in 10 patients and Polymorphic VT in one patient. Other patient issues included damage to leaflets (n=7, 15.6%) which necessitated surgery in one case and prompted consideration of the same in another. There were 4 reports of clip entrapment in the chordae. Device-related issues included 3 cases of leaks in the steerable guide catheter affecting its ability to hold the column, knotting on the lock line, difficulties with positioning the second clip above the valve, clip reopening beyond the expected 5°, clip opening while locked but staying closed post-deployment, delays in clip delivery, and challengers in guiding catheter positioning. No acute deaths were reported in the MAUDE database within 2 months of device approval.
Conclusion:
Our research findings summarize the reported adverse events during the early period following FDA approval of Triclip G4 T-TEER system. This provides valuable insights into common failure modes and complications, offering guidance on their optimal utilization. Multiple adverse events can be noted soon after approval of the Triclip, underscoring the importance of good initial training and proctoring.
More abstracts on this topic:
Angiography-derived FFR-Guided Coronary Artery Bypass Grafting for Patients undergoing Valve Surgery with Concomitant Coronary Artery Disease (FAVOR 4-QVAS): a Randomized Trial
Zhu Yunpeng, Guo Zhigang, Zhu Dan, Zhang Xiquan, Chen Liangwan, Redfors Bjorn, Sandner Sigrid, Gaudino Mario, Tu Shengxian, Zhao Qiang, Cheng Zhaoyun, Zhao Yuan, Zhang Wei, Han Lin, Zhang Chengxin, Yang Sumin, Ma Liang, Qiao Chenhui
Cryptogenic stroke associated with a double interatrial septum treated with surgical closureNabi Michelle, Jeha Jeannine, Walker Jennifer, Mahadevan Vaikom, Rastogi Ujjwal