Final ID: Mo1059
Diverse Morphology and Proteomic Phenotypes of Calcification in Bioprosthetic Structural Valve Degeneration
Abstract Body (Do not enter title and authors here): Introduction: Fibrocalcific remodeling is an end-stage feature of bioprosthetic (BP) structural valve degeneration and calcific aortic valve (AV) disease. However, the processes governing BP calcification are understudied. Here we conduct a histopathological assessment of BP degeneration in the aortic position and build a proteomic comparison map of BP degeneration versus AV disease in humans.
Methods: Macroscopic segmentation was performed on entire explanted degenerated bovine pericardial BP leaflets (n=48) and diseased AV valves (n=19) and validated with histology to classify their state (BP: non-degenerated/thrombotic/neotissue/calcified, AV: non-diseased/fibrotic/calcified). Segment-specific bulk mass spectrometry-based proteomics was performed on BP/AV tissues. Laser capture microdissection enabled spatially resolved proteomics of BP calcification within discrete bioprosthetic/thrombotic/neotissue matrices versus explanted non-calcified regions.
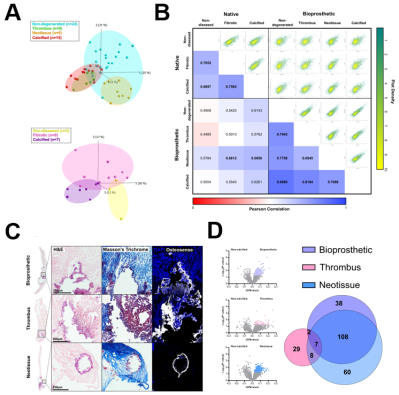
Results: Principal component analysis revealed BP and AV proteomes (2,005 and 2,012 proteins) clustered according to their degenerated and diseased segments; BP thrombotic and BP calcified sample clusters overlapped (Fig.A). Correlations of segment proteome-wide abundances revealed the highest intra-tissue similarity between non-degenerated BP and calcified BP (rp=0.87). The highest inter-tissue similarity was found between BP neotissue and calcified AV (rp=0.69) (Fig.B). Histopathological observations supported these proteomic findings, confirming the presence of calcification within discrete BP matrices: bioprosthetic (22/59), thrombus (22/59), and neotissue (15/59) (Fig.C). Laser capture microdissection revealed that 252 proteins were enriched in either bioprosthetic, thrombotic, or neotissue matrix calcification compared to explanted non-calcified regions. Only 3% of differentially enriched proteins overlapped, suggesting different mechanisms (Fig.D).
Conclusions: This is the first comparative proteomic study of segmented degenerated BP and diseased AV tissue. We identified 3 subtypes of BP calcification within bioprosthetic, thrombotic, or neotissue matrices. Spatial proteomics revealed different proteins associated with the calcification of these matrices.
Methods: Macroscopic segmentation was performed on entire explanted degenerated bovine pericardial BP leaflets (n=48) and diseased AV valves (n=19) and validated with histology to classify their state (BP: non-degenerated/thrombotic/neotissue/calcified, AV: non-diseased/fibrotic/calcified). Segment-specific bulk mass spectrometry-based proteomics was performed on BP/AV tissues. Laser capture microdissection enabled spatially resolved proteomics of BP calcification within discrete bioprosthetic/thrombotic/neotissue matrices versus explanted non-calcified regions.
Results: Principal component analysis revealed BP and AV proteomes (2,005 and 2,012 proteins) clustered according to their degenerated and diseased segments; BP thrombotic and BP calcified sample clusters overlapped (Fig.A). Correlations of segment proteome-wide abundances revealed the highest intra-tissue similarity between non-degenerated BP and calcified BP (rp=0.87). The highest inter-tissue similarity was found between BP neotissue and calcified AV (rp=0.69) (Fig.B). Histopathological observations supported these proteomic findings, confirming the presence of calcification within discrete BP matrices: bioprosthetic (22/59), thrombus (22/59), and neotissue (15/59) (Fig.C). Laser capture microdissection revealed that 252 proteins were enriched in either bioprosthetic, thrombotic, or neotissue matrix calcification compared to explanted non-calcified regions. Only 3% of differentially enriched proteins overlapped, suggesting different mechanisms (Fig.D).
Conclusions: This is the first comparative proteomic study of segmented degenerated BP and diseased AV tissue. We identified 3 subtypes of BP calcification within bioprosthetic, thrombotic, or neotissue matrices. Spatial proteomics revealed different proteins associated with the calcification of these matrices.
More abstracts on this topic:
A Novel Role for ANPEP in Osteogenic Signaling and Aortic Valve Calcification
Fan Jianing, Chen Qixin, Miao Jiaxin, Li Zhenzhen, Zhou Daxin, Ge Junbo
Association between Secondary Mitral Regurgitation and Left Atrial Structural Abnormalities in Atrial FibrillationKassar Ahmad, Chamoun Nadia, Chahine Yaacoub, Akoum Nazem