Final ID: MDP1581
SEX DISPARITIES IN HUMAN CALCIFIC AORTIC VALVE STENOSIS ASSESSED BY DISEASE STAGE-SPECIFIC HISTOPATHOLOGICAL AND PROTEOMIC ANALYSES
Abstract Body (Do not enter title and authors here): BACKGROUND:
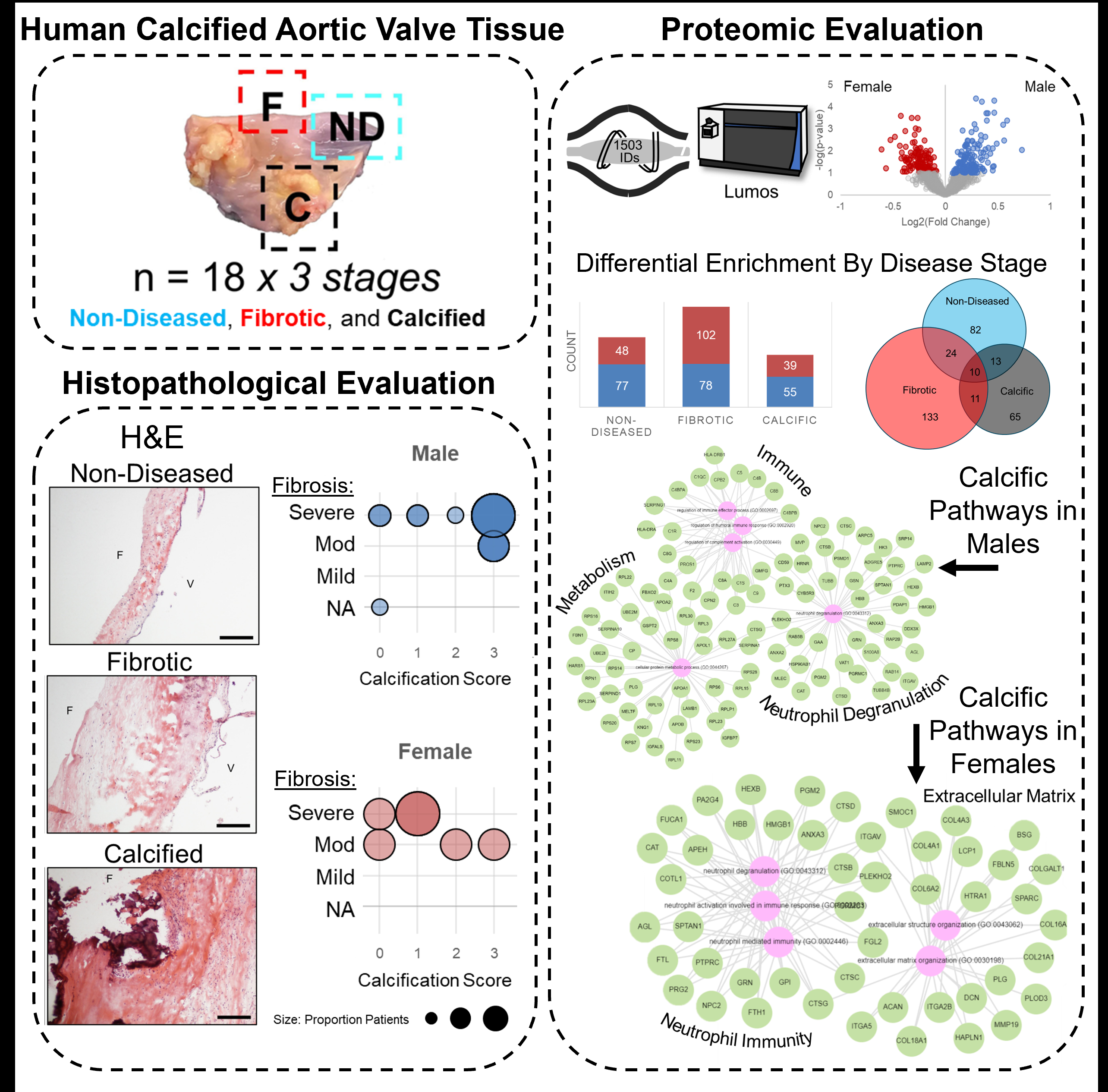
Calcific aortic valve stenosis (CAVS) is a global clinical burden, impacting around 2% of the population over 65 years of age. No pharmacotherapeutics exist, with surgical repair and transcatheter valve replacement being the only intervention. Females are underrepresented in studies of CAVS, leading to delay in timely intervention and increased mortality. Histopathology demonstrates female CAVS presents with decreased valvular calcification but increased fibrosis and severity of symptoms. We hypothesize that the underlying molecular mechanisms contributing to disease progression and fibrocalcific burden in AS differs between male and female patients. Our goal for this study is to use previously acquired proteomic datasets of a clinically-defined human AS cohort to examine sex disparities and underlying sex-specific disease signatures.
METHODS and RESULTS:
Age-matched human AS tissue samples (n=4 females, n=14 males) were each segmented into non-diseased, fibrotic, and calcified disease stages and analyzed using LC-MS/MS proteomics and quantitative histopathology. Unbiased principal component analysis shows sex- and stage-specific proteome clustering. AS pathogenesis drove sex-specific disparities in the valvular proteome: 338/1503 total proteins were differentially-enriched by sex across disease stages. Compared to sex-specific non-diseased controls, female fibrotic tissue resulted in 2.05-fold greater number of differentially-enriched proteins than did male fibrotic tissue (female: 113, male: 55; q<0.05 threshold). In contrast, female calcific tissue identified 2.03-fold less differentially-enriched proteins than male calcific tissue (female: 255, male 519; q<0.05 threshold). By Gene Ontology enrichment (odds ratio ranked, q<0.05), proteins enriched in male fibrotic AS segments preferentially associate with superoxide regulation, sterol transport, and endocytosis while extracellular matrix (ECM) organization and endothelial cell migration were hallmarks of female-specific AS fibrosis. Remarkably, immune and complement response were ubiquitous drivers of calcification, female segments were uniquely enriched in ECM processes.
CONCLUSIONS:
We reveal a sexually-dimorphic AS proteome, including the novel overabundance of ECM remodeling pathways in female calcified aortic valve tissues. This analysis allows for identification of potential sex-specific protein drug targets implicated in AS pathobiology.
Calcific aortic valve stenosis (CAVS) is a global clinical burden, impacting around 2% of the population over 65 years of age. No pharmacotherapeutics exist, with surgical repair and transcatheter valve replacement being the only intervention. Females are underrepresented in studies of CAVS, leading to delay in timely intervention and increased mortality. Histopathology demonstrates female CAVS presents with decreased valvular calcification but increased fibrosis and severity of symptoms. We hypothesize that the underlying molecular mechanisms contributing to disease progression and fibrocalcific burden in AS differs between male and female patients. Our goal for this study is to use previously acquired proteomic datasets of a clinically-defined human AS cohort to examine sex disparities and underlying sex-specific disease signatures.
METHODS and RESULTS:
Age-matched human AS tissue samples (n=4 females, n=14 males) were each segmented into non-diseased, fibrotic, and calcified disease stages and analyzed using LC-MS/MS proteomics and quantitative histopathology. Unbiased principal component analysis shows sex- and stage-specific proteome clustering. AS pathogenesis drove sex-specific disparities in the valvular proteome: 338/1503 total proteins were differentially-enriched by sex across disease stages. Compared to sex-specific non-diseased controls, female fibrotic tissue resulted in 2.05-fold greater number of differentially-enriched proteins than did male fibrotic tissue (female: 113, male: 55; q<0.05 threshold). In contrast, female calcific tissue identified 2.03-fold less differentially-enriched proteins than male calcific tissue (female: 255, male 519; q<0.05 threshold). By Gene Ontology enrichment (odds ratio ranked, q<0.05), proteins enriched in male fibrotic AS segments preferentially associate with superoxide regulation, sterol transport, and endocytosis while extracellular matrix (ECM) organization and endothelial cell migration were hallmarks of female-specific AS fibrosis. Remarkably, immune and complement response were ubiquitous drivers of calcification, female segments were uniquely enriched in ECM processes.
CONCLUSIONS:
We reveal a sexually-dimorphic AS proteome, including the novel overabundance of ECM remodeling pathways in female calcified aortic valve tissues. This analysis allows for identification of potential sex-specific protein drug targets implicated in AS pathobiology.
More abstracts on this topic:
β-1 Adrenoceptor is Responsible for the Apamin-Sensitive Small Conductance Ca2+-Activated K+ Current Activation in Female Rabbit Ventricles
Yang Minjing, Zhang Liyang, Kote Anxhela, Tisdale James, Chen Zhenhui, Everett Thomas, Chen Peng-sheng, Liu Xiao
Balloon-expandable versus Self-expandable transcatheter aortic valve replacement for failed surgical prostheses: A systematic review and meta-analysisK. Awad Ahmed, Khalefa Basma, Negmeldin Aly Yassin Mazen, Arnaout Moumen, Mostafa Naydeen, S. Naeem Mariana, Eljadid Ghaith, Gardezi Syed Karam