Final ID: MDP1656
Variation in Vasoactive Treatments for Cardiogenic Shock: Insights from the Critical Care Cardiology Trials Network
Abstract Body (Do not enter title and authors here): Introduction: The paucity of data to guide selection of specific vasoactive agents in patients with cardiogenic shock (CS) may lead to variability in practice patterns.
Hypothesis: Variation in the utilization of inodilators to treat CS is associated with both institution- and patient-level factors.
Methods: The Critical Care Cardiology Trials Network (CCCTN) is a multicenter network of tertiary CICUs in North America coordinated by the TIMI Study Group. This analysis cohort includes patients with CS from 2018-2023. Shock was defined as systolic blood pressure less than 90 mmHg with end-organ dysfunction ascribed to the hypotension. Shock type was classified by site investigators. Multivariable linear mixed-effect modeling was used to evaluate patient- and institutional-level variation associated with the use of inodilators treatment (milrinone, dobutamine).
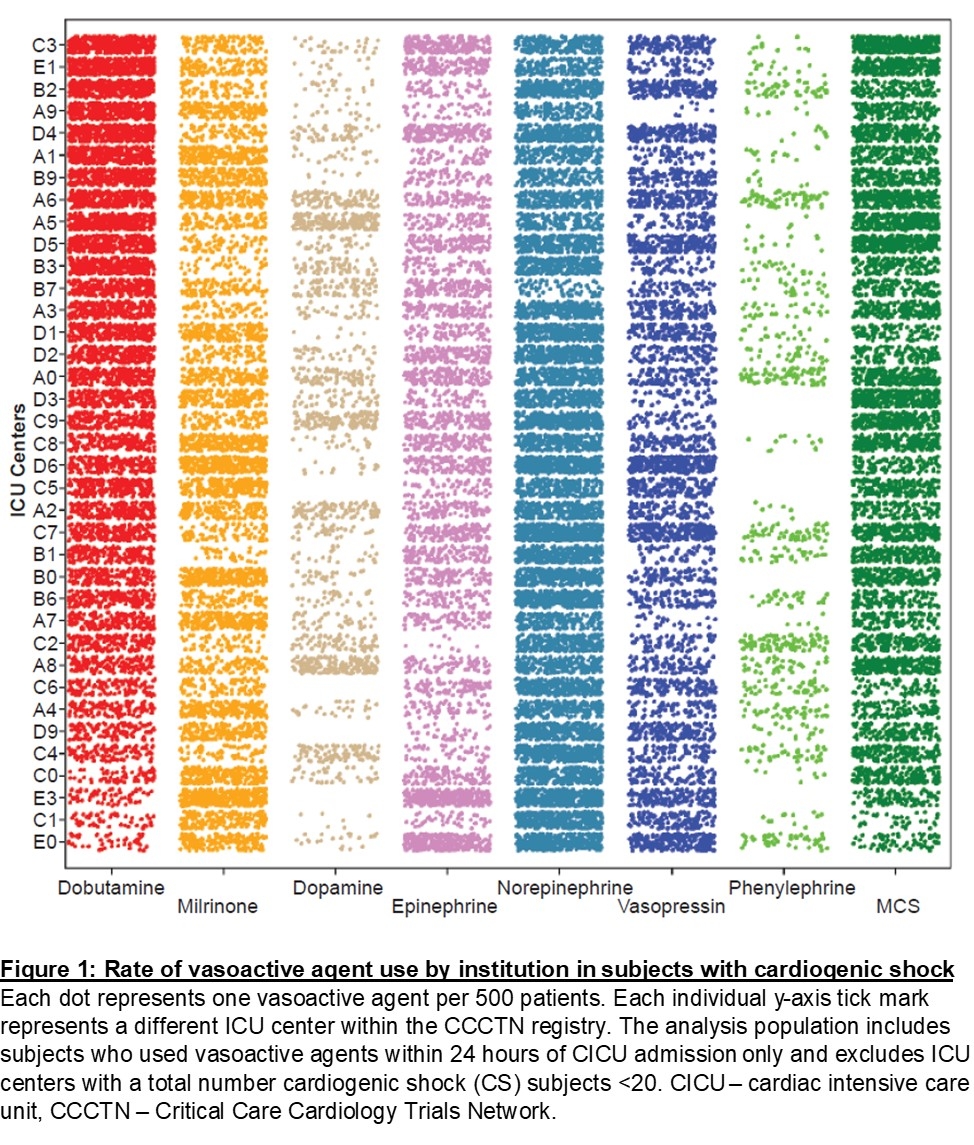
Results: A total of 3,282 patients from 37 CICUs were in the study cohort. The rate of utilization of specific vasoactive medications and MCS varied substantially across institutions (Fig 1). For inodilators, rates of dobutamine use varied by institution from 10% to 82%, while milrinone use varied from 6% to 71%. Patient-level variables that were associated with the use of inodilator treatment included male sex (OR 1.24 [95% CI 1.03-1.49]), history of heart failure (1.98 [1.61-2.43]), SCAI class D vs. B/C (1.35 [1.08-1.69]), and BiV failure vs. LV failure (1.59 [1.27-1.99). Variables less likely to be associated with inodilator use included age (0.97 [0.97-0.98] per year increase), cardiac arrest (0.34 [0.27-0.42]), and acute coronary syndrome presentation (0.72 [0.57-0.91]). MCS use within 36 hours of CICU admission and pulmonary hypertension were not associated with variability in inodilator use. In multivariable linear mixed-effect modeling, 45.9% of variation in inodilator use was attributed to patient-level factors and 21.8% of variation was attributed to the institution.
Conclusion: There is substantial variation in vasoactive treatment and inodilator use related to patient- and institution-level factors. Such variability underscores the need for additional high-quality evidence to guide vasoactive treatment strategies in CS.
Hypothesis: Variation in the utilization of inodilators to treat CS is associated with both institution- and patient-level factors.
Methods: The Critical Care Cardiology Trials Network (CCCTN) is a multicenter network of tertiary CICUs in North America coordinated by the TIMI Study Group. This analysis cohort includes patients with CS from 2018-2023. Shock was defined as systolic blood pressure less than 90 mmHg with end-organ dysfunction ascribed to the hypotension. Shock type was classified by site investigators. Multivariable linear mixed-effect modeling was used to evaluate patient- and institutional-level variation associated with the use of inodilators treatment (milrinone, dobutamine).
Results: A total of 3,282 patients from 37 CICUs were in the study cohort. The rate of utilization of specific vasoactive medications and MCS varied substantially across institutions (Fig 1). For inodilators, rates of dobutamine use varied by institution from 10% to 82%, while milrinone use varied from 6% to 71%. Patient-level variables that were associated with the use of inodilator treatment included male sex (OR 1.24 [95% CI 1.03-1.49]), history of heart failure (1.98 [1.61-2.43]), SCAI class D vs. B/C (1.35 [1.08-1.69]), and BiV failure vs. LV failure (1.59 [1.27-1.99). Variables less likely to be associated with inodilator use included age (0.97 [0.97-0.98] per year increase), cardiac arrest (0.34 [0.27-0.42]), and acute coronary syndrome presentation (0.72 [0.57-0.91]). MCS use within 36 hours of CICU admission and pulmonary hypertension were not associated with variability in inodilator use. In multivariable linear mixed-effect modeling, 45.9% of variation in inodilator use was attributed to patient-level factors and 21.8% of variation was attributed to the institution.
Conclusion: There is substantial variation in vasoactive treatment and inodilator use related to patient- and institution-level factors. Such variability underscores the need for additional high-quality evidence to guide vasoactive treatment strategies in CS.
More abstracts on this topic:
6-Nitrodopamine potentiates the positive chronotopic and inotropic effect induced by noradrenaline in the rat isolated heart
Lima Antonio, Sobanski Joao Fernando, Antunes Edson, De Nucci Gilberto
Association between Pressure-Adjusted Heart Rate and Mortality in Cardiogenic ShockGinder Curtis, Jentzer Jacob, Guo Jianping, Van Diepen Sean, Katz Jason, Morrow David, Berg David