Final ID: Mo2127
Colchicine for prevention of pericarditis after catheter ablation: A systematic review and meta-analysis.
Abstract Body (Do not enter title and authors here):
Introduction: Catheter ablation (CA) therapy is well established therapy for different types of atrial fibrillation, with some concern including post ablation pericarditis which increases the morbidity and mortality. Colchicine, known for its anti-inflammatory effects has shown to be effective in reducing the incidence of perioperative AF after cardiac surgery. However, the efficacy of post ablation colchicine is still debatable. We aimed to establish evidence on the use of colchicine for preventing pericarditis after catheter ablation.
Methods: We searched PubMed, Scopus, WOS and Cochrane until May 2024 for relevant studies that assessed colchicine after CA. The primary outcome of interest was the incidence of pericarditis following AF. Other secondary outcomes were the incidence of pericardial effusion, GI adverse events, and hospitalization rates.
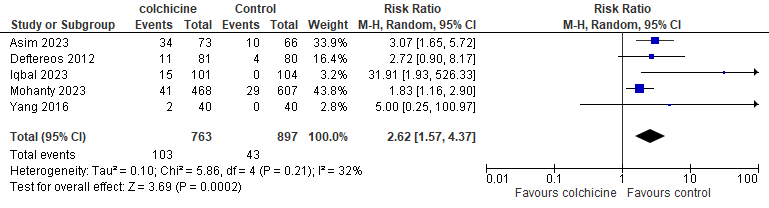
Results: A total of nine studies were included with a total of 2795 patients aged 66 to 69.4 years, Colchicine administration before catheter ablation showed a significant decrease in the occurrence of clinical pericarditis after AF ablation compared to placebo (RR=0.38; 95% CI: 0.27, 0.53). Moreover, colchicine was favored to decrease the incidence of AF recurrence rate at 3, 6, and 12 months with the following values, respectively (RR=0.58, 95% CI: 0.42 to 0.80, 0.69, 95% CI: 0.5 to 0.97, and 0.76, 95% CI: 0.66 to 0.87). On the other hand, colchicine was associated with a significant increase in GI adverse events (RR= 2.62, 95% CI: 1.57, 4.37).
Conclusion: Colchicine was found to be a promising intervention for reducing the incidence of pericarditis following atrial fibrillation ablation. Despite some contradictory data about gastrointestinal side effects, cautious dosage measures, such as weight-adjusted doses, may improve tolerability while maintaining efficacy in reducing post-ablation events.
Introduction: Catheter ablation (CA) therapy is well established therapy for different types of atrial fibrillation, with some concern including post ablation pericarditis which increases the morbidity and mortality. Colchicine, known for its anti-inflammatory effects has shown to be effective in reducing the incidence of perioperative AF after cardiac surgery. However, the efficacy of post ablation colchicine is still debatable. We aimed to establish evidence on the use of colchicine for preventing pericarditis after catheter ablation.
Methods: We searched PubMed, Scopus, WOS and Cochrane until May 2024 for relevant studies that assessed colchicine after CA. The primary outcome of interest was the incidence of pericarditis following AF. Other secondary outcomes were the incidence of pericardial effusion, GI adverse events, and hospitalization rates.
Results: A total of nine studies were included with a total of 2795 patients aged 66 to 69.4 years, Colchicine administration before catheter ablation showed a significant decrease in the occurrence of clinical pericarditis after AF ablation compared to placebo (RR=0.38; 95% CI: 0.27, 0.53). Moreover, colchicine was favored to decrease the incidence of AF recurrence rate at 3, 6, and 12 months with the following values, respectively (RR=0.58, 95% CI: 0.42 to 0.80, 0.69, 95% CI: 0.5 to 0.97, and 0.76, 95% CI: 0.66 to 0.87). On the other hand, colchicine was associated with a significant increase in GI adverse events (RR= 2.62, 95% CI: 1.57, 4.37).
Conclusion: Colchicine was found to be a promising intervention for reducing the incidence of pericarditis following atrial fibrillation ablation. Despite some contradictory data about gastrointestinal side effects, cautious dosage measures, such as weight-adjusted doses, may improve tolerability while maintaining efficacy in reducing post-ablation events.
More abstracts on this topic:
A Rare Case of Adalimumab-Induced Cardiac Tamponade in a Patient with Psoriatic Arthritis
Raval Akhinav, Tran Minh, Saini Ishveen, Rea Mark
An Inflammatory Dilemma: Human Metapneumovirus-Associated Pericarditis in a Solitary Kidney HostRethnaswamy Sherry, King Lauren, Benson Christopher