Final ID: Su2138
Heritable heart failure traits in mice undergoing early life stress
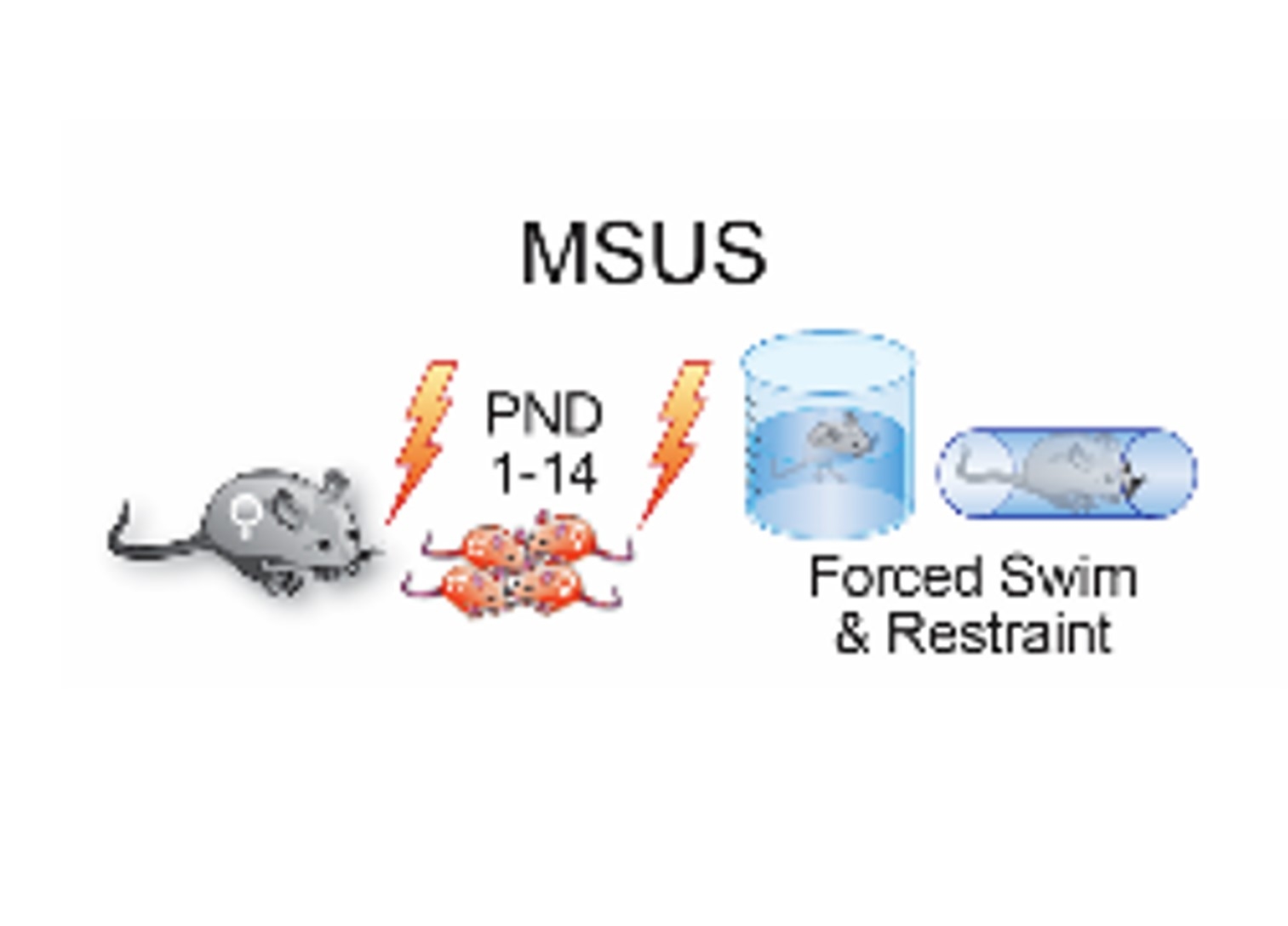
Abstract Body (Do not enter title and authors here): Introduction:Adverse childhood experiences, also known as early life stress (ELS), are associated with increased risk of cardiovascular disease in later life, yet the underlying mechanisms remain elusive. Recent evidence indicates that parental life experiences can be transmitted to the offspring. Aim:To investigate the effects of ELS on cardiac structure and function in exposed parents and in their offspring, across 3 generations. Methods:We used ELS mouse model based on unpredictable separation of mouse pups (F1) from their mother (F0) each day for 3 hours from postnatal day 1 (PND1) to PND14 combined with dams exposure to an additional unpredictable stressor (forced swim in 18°C water for 5 minutes or 20-minute physical restraint in a tube) during separation. Control litters were raised normally. Echocardiography was performed at 6, 12 and 18 months in exposed animals (F0), their unexposed offspring (F1) and grand-offspring (F2). Both male and female mice were studied. Heart weight/tibia length was used to assess cardiac mass while Masson's Trichrome was employed to detect fibrosis. Lung congestion was assessed as lung wet/dry weight ratio. Single-cell RNA sequencing (scRNAseq) was performed in MSUS and control hearts. A 6-week environmental enrichment (EE) program (cages containing running wheels, maze) was employed to test the possible rescue of ELS effects in adult males and their offspring. Results:F1 MSUS mice displayed increased LV mass, impaired diastolic function (assessed by conventional and tissue Doppler analysis) myocardial fibrosis and lung congestion. Time-dependent worsening of cardiac performance was observed from 6 to 18 months, both in males and females. ScRNAseq unveiled dysregulation of transcriptional programs underlying inflammation and lipotoxicity in the cardiomyocyte and endothelial cell clusters. MSUS offsprings did not show changes of cardiac function at 6 months, however diastolic dysfunction and lung congestion were observed at 12 and 18 months. A similar impairment of cardiac function was observed in the MSUS grandoffspring (F3). Of interest, 6-week exposure to an environmental enrichment protocol was able to improve LV mass, diastolic function and lung congestion in 12 months-old MSUS mice.
Conclusions: ELS induces a transgenerational transmission of cardiac phenotypic alterations which can be rescued by EE. Our results shed light on the potential role of ELS on heart failure development and potential mitigation strategies.
Conclusions: ELS induces a transgenerational transmission of cardiac phenotypic alterations which can be rescued by EE. Our results shed light on the potential role of ELS on heart failure development and potential mitigation strategies.
More abstracts on this topic:
Amusement Park Rides and Cardiac Devices: Heart Dropper or Heart Stopper?
Johnson Alexander, Kassab Mia, Kallas Dania, Franciosi Sonia, Bone Jeffrey, Sanatani Shubhayan
A20 in the Kidney Epithelium Attenuates Angiotensin II-induced Hypertension by Constraining Renal Tubular NHE3 ExpressionLu Xiaohan, Ren Jiafa, Wen Yi, Griffiths Robert, Yang Ting, Hammer Gianna, Zhuo Jia, Crowley Steven