Final ID: 4140257
CDCP1’s Role in Cardiac Fibrosis: Unveiling Potential Mechanisms in a Pressure Over-Load Mouse Model
Abstract Body (Do not enter title and authors here): Background: CDCP1 has been associated with reverse remodeling in human dilated cardiomyopathy (DCM) mediating its effect by reducing cardiac fibrosis which has been demonstrated in-vitro using human cardiac fibroblasts (CF) and in-vivo histologically in mice. However, the role of and the molecular mechanisms by which CDCP1 attenuates cardiac fibrosis in-vivo is unknown.
Methods: To characterize the transcriptomic profiles of CDCP1 in cardiac fibrosis, Cdcp1 KO FVB/NJ mice were generated, and implanted with osmotic minipumps containing angiotensin II and phenylephrine (Ang II/PE) or saline at age of 10 weeks. There were 4 experimental groups (6 mice each), Saline_WT, Saline_KO, AngII/PE_WT, and AngII/PE_KO. After 4 weeks of AngII/PE induction, mice were euthanized, RNA was extracted from their heart tissue followed by RNA-seq to explore transcriptomic profiles. Fibrosis was histologically determined using picosirius (PSR) staining and was quantified by percentage of fibrosis area.
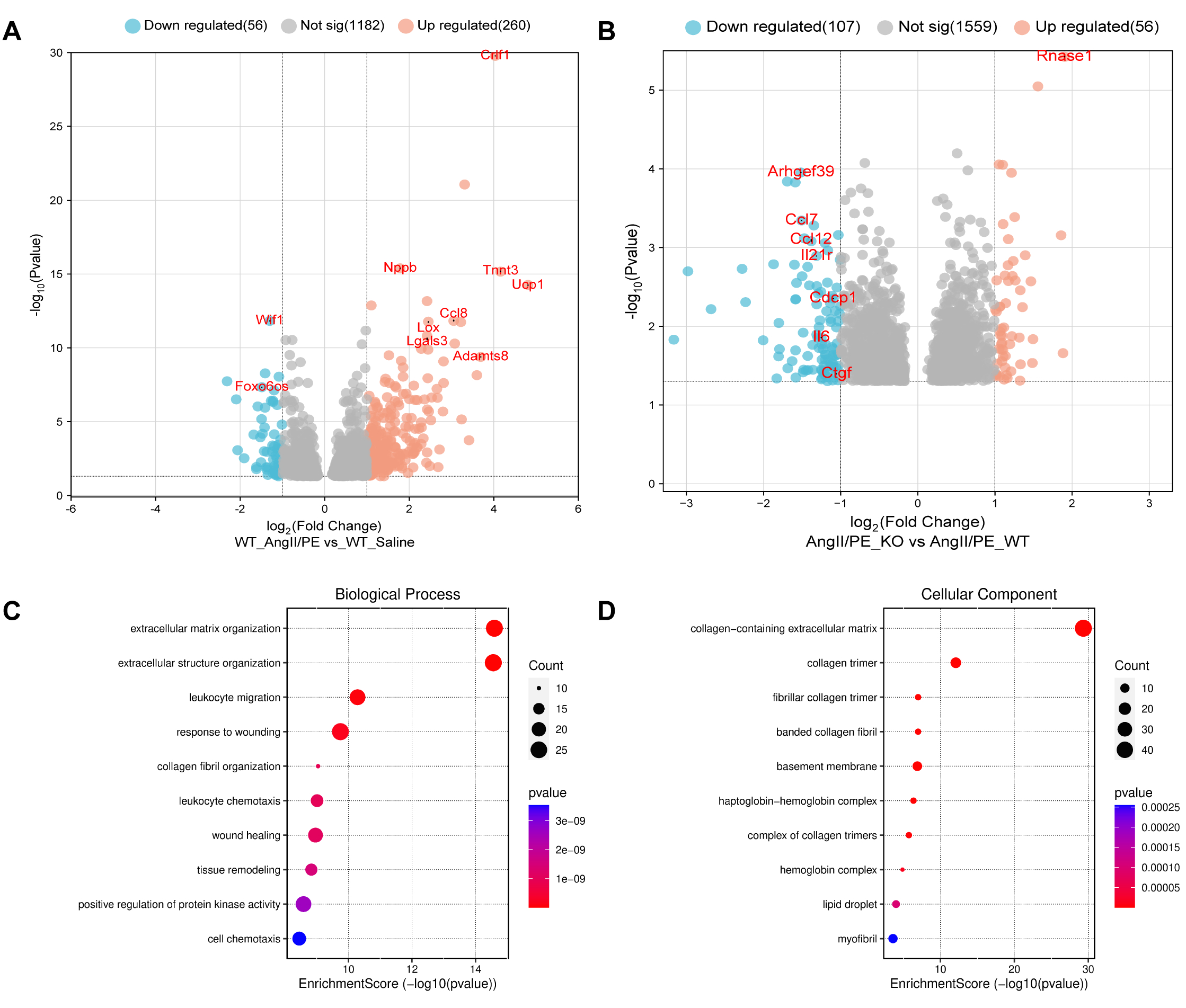
Results: Histological analysis demonstrated that Cdcp1 KO attenuated severe cardiac fibrosis by 32.7% determined by quantification of percentage of fibrosis area. When comparing the transcriptome-wide gene expression in WT mice hearts from Saline to AngII/PE induction, a total of 316 differentially expressed genes (DEGs) were identified (Fig. 1A). Expression of Nppb (encodes ProBNP), a marker for heart failure, was significantly upregulated. The most significantly upregulated DEG after AngII/PE induction was Crlf1 (Fig. 1A), a gene predominantly expressed in CF, consistent with previous findings. We next compared transcriptomic profiles of AngII/PE induction WT and Cdcp1 KO mice hearts, to explore the role of CDCP1 in cardiac fibrosis. A total of 163 DEGs were identified (Fig. 1B). Notably, Cdcp1-mediated DEGs enriched GO Pathways associated with extracellular matrix organization (Biological Process) and collagen-containing extracellular matrix (Cellular Component) (Figs. 1C, 1D), pivotal processes in cardiac fibrosis. In addition, Cdcp1 KO resulted in several “top” DEGs (including Rnase1, Ccl7, Ccl12 Il21r, and Il6) which have known function in inflammation, suggesting that CDCP1 might attenuate cardiac fibrosis through immune regulation .
Conclusion: Our findings underscore the pivotal role of CDCP1 in modulating cardiac fibrosis in-vivo, potentially through immune regulation. Targeting CDCP1 may offer promising therapeutic avenues for mitigating myocardial fibrosis.
Methods: To characterize the transcriptomic profiles of CDCP1 in cardiac fibrosis, Cdcp1 KO FVB/NJ mice were generated, and implanted with osmotic minipumps containing angiotensin II and phenylephrine (Ang II/PE) or saline at age of 10 weeks. There were 4 experimental groups (6 mice each), Saline_WT, Saline_KO, AngII/PE_WT, and AngII/PE_KO. After 4 weeks of AngII/PE induction, mice were euthanized, RNA was extracted from their heart tissue followed by RNA-seq to explore transcriptomic profiles. Fibrosis was histologically determined using picosirius (PSR) staining and was quantified by percentage of fibrosis area.
Results: Histological analysis demonstrated that Cdcp1 KO attenuated severe cardiac fibrosis by 32.7% determined by quantification of percentage of fibrosis area. When comparing the transcriptome-wide gene expression in WT mice hearts from Saline to AngII/PE induction, a total of 316 differentially expressed genes (DEGs) were identified (Fig. 1A). Expression of Nppb (encodes ProBNP), a marker for heart failure, was significantly upregulated. The most significantly upregulated DEG after AngII/PE induction was Crlf1 (Fig. 1A), a gene predominantly expressed in CF, consistent with previous findings. We next compared transcriptomic profiles of AngII/PE induction WT and Cdcp1 KO mice hearts, to explore the role of CDCP1 in cardiac fibrosis. A total of 163 DEGs were identified (Fig. 1B). Notably, Cdcp1-mediated DEGs enriched GO Pathways associated with extracellular matrix organization (Biological Process) and collagen-containing extracellular matrix (Cellular Component) (Figs. 1C, 1D), pivotal processes in cardiac fibrosis. In addition, Cdcp1 KO resulted in several “top” DEGs (including Rnase1, Ccl7, Ccl12 Il21r, and Il6) which have known function in inflammation, suggesting that CDCP1 might attenuate cardiac fibrosis through immune regulation .
Conclusion: Our findings underscore the pivotal role of CDCP1 in modulating cardiac fibrosis in-vivo, potentially through immune regulation. Targeting CDCP1 may offer promising therapeutic avenues for mitigating myocardial fibrosis.
More abstracts on this topic:
4-Hydroxy-2-Nonenal Alters Alternative Polyadenylation to Regulate mRNA Isoform Diversity in the Transition from Human Cardiac Fibroblasts to Myofibroblasts
Natarajan Kartiga, Neupane Rahul, Yalamanchili Hari Krishna, Palaniyandi Suresh, Wagner Eric, Guha Ashrith, Amirthalingam Thandavarayan Rajarajan
A Case of Immune Checkpoint Inhibitor-Induced Myocarditis with Myositis and/or Myasthenia Gravis Overlap Syndrome with New Right Bundle Branch Block and Negative Cardiac MRIDasari Srikiran