Final ID: MDP470
Short- and long-term impact of aspirin cessation in older adults: a target trial emulation.
Abstract Body (Do not enter title and authors here): Background: The net benefit of aspirin cessation in older adults remains uncertain. This study aimed to use observational data to emulate a randomized trial of aspirin cessation versus continuation in older adults without cardiovascular disease (CVD).
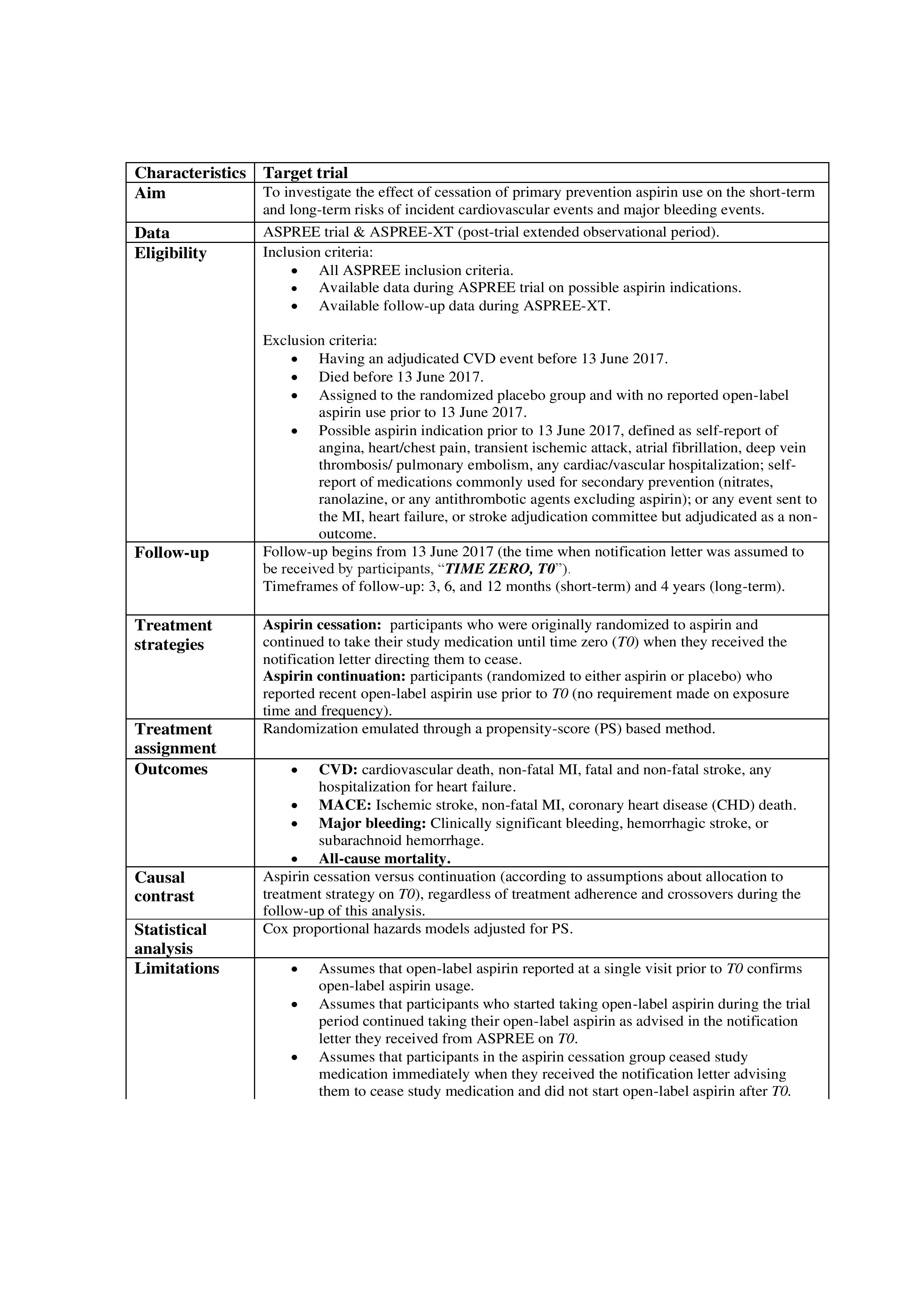
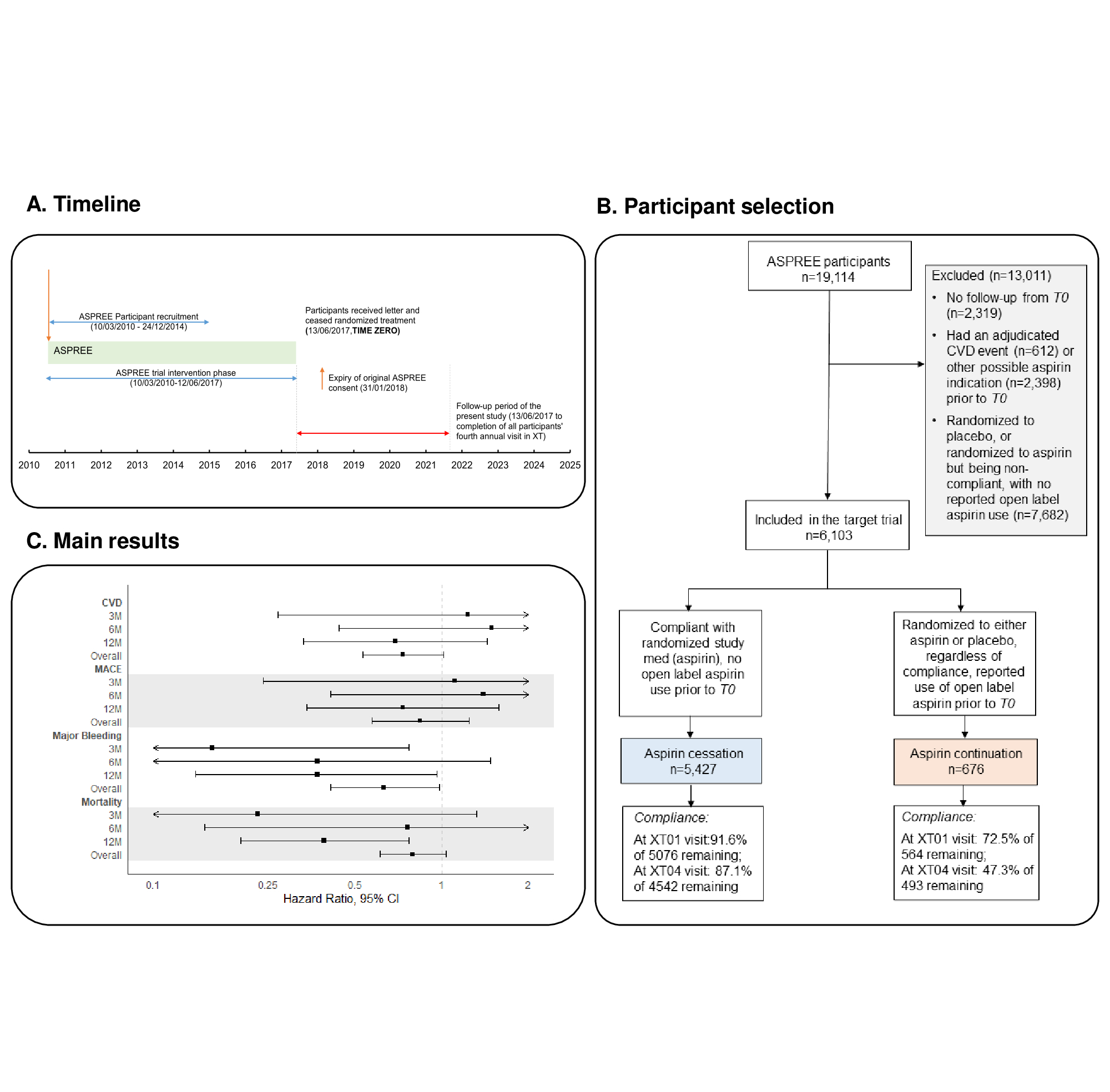
Methods: Post-hoc analysis using a target trial emulation framework (Table 1) applied to the immediate post-trial period (2017-2021) of a study of low-dose aspirin initiation in 19,114 adults aged 70 years and older (ASPREE; NCT01038583). Participants from Australia and US were included if they were free of CVD at the start of the post-trial intervention period (time zero, T0) and had been taking open-label or randomized aspirin immediately before T0 (Fig 1A). The two groups in the target trial were: aspirin cessation (participants who were taking randomized aspirin immediately before T0; assumed to have stopped at T0 as instructed) versus aspirin continuation (participants on open-label aspirin at T0 regardless of their randomized treatment; assumed to have continued at T0). The outcomes after T0 were incident CVD, major adverse cardiovascular events (MACE), all-cause mortality, and major bleeding during 3, 6, and 12 months (short-term), and 48 months (long-term) follow-up. Hazard ratios (HRs) comparing aspirin cessation to continuation were estimated from propensity-score (PS) adjusted Cox proportional-hazards regression models.
Results: We included 6,103 CVD-free participants (cessation: 5,427, continuation: 676). Participant selection flow chart is presented in Fig 1B. Over both short- and long-term follow-up, aspirin cessation versus continuation was not associated with elevated risk of CVD, MACE and all-cause mortality (HRs, at 3 and 48 months respectively were, 1.23 and 0.73 for CVD; 1.11 and 0.84 for MACE; 0.23 and 0.79 for all-cause mortality, p >0.05) but cessation had a reduced risk of incident major bleeding events (HRs at 3 and 48 months, 0.16 and 0.63, p <0.05) (Fig 1C). Similar findings were seen for all outcomes at 6 and 12 months, except for a lowered risk of all-cause mortality in the cessation group at 12 months (Fig 1C).
Conclusions: Our findings support the safety of deprescribing prophylactic aspirin used for primary prevention in older adults.
Methods: Post-hoc analysis using a target trial emulation framework (Table 1) applied to the immediate post-trial period (2017-2021) of a study of low-dose aspirin initiation in 19,114 adults aged 70 years and older (ASPREE; NCT01038583). Participants from Australia and US were included if they were free of CVD at the start of the post-trial intervention period (time zero, T0) and had been taking open-label or randomized aspirin immediately before T0 (Fig 1A). The two groups in the target trial were: aspirin cessation (participants who were taking randomized aspirin immediately before T0; assumed to have stopped at T0 as instructed) versus aspirin continuation (participants on open-label aspirin at T0 regardless of their randomized treatment; assumed to have continued at T0). The outcomes after T0 were incident CVD, major adverse cardiovascular events (MACE), all-cause mortality, and major bleeding during 3, 6, and 12 months (short-term), and 48 months (long-term) follow-up. Hazard ratios (HRs) comparing aspirin cessation to continuation were estimated from propensity-score (PS) adjusted Cox proportional-hazards regression models.
Results: We included 6,103 CVD-free participants (cessation: 5,427, continuation: 676). Participant selection flow chart is presented in Fig 1B. Over both short- and long-term follow-up, aspirin cessation versus continuation was not associated with elevated risk of CVD, MACE and all-cause mortality (HRs, at 3 and 48 months respectively were, 1.23 and 0.73 for CVD; 1.11 and 0.84 for MACE; 0.23 and 0.79 for all-cause mortality, p >0.05) but cessation had a reduced risk of incident major bleeding events (HRs at 3 and 48 months, 0.16 and 0.63, p <0.05) (Fig 1C). Similar findings were seen for all outcomes at 6 and 12 months, except for a lowered risk of all-cause mortality in the cessation group at 12 months (Fig 1C).
Conclusions: Our findings support the safety of deprescribing prophylactic aspirin used for primary prevention in older adults.
More abstracts on this topic:
Abdominal Circumference and Coronary Calcium Score in a Healthy Nonobese Brazilian Cohort: ELSA-Brasil Cohort Analysis
Correa Fabiano Ronaldo, Bittencourt Marcio, Bosco Mendes Thiago, Romero-nunez Carlos, Generoso Giuliano, Staniak Henrique, Foppa Murilo, Santos Raul, Lotufo Paulo, Bensenor Isabela
An Inflammatory Dilemma: Human Metapneumovirus-Associated Pericarditis in a Solitary Kidney HostRethnaswamy Sherry, King Lauren, Benson Christopher