Final ID: Su3032
Changes in high-sensitivity cardiac troponin I and associated cardiovascular risk: Analyses From the FOURIER Trial
Abstract Body (Do not enter title and authors here): Background: Circulating high-sensitivity cardiac troponin I (hs-cTnI) is associated with the risk of future cardiovascular (CV) events in stable patients (pts). Data are scarce regarding changes of hs-cTnI in a stable setting.
Aims: To study baseline and changes of hs-cTnI in pts with atherosclerotic CV disease (ASCVD), and the association with risk of major CV events.
Methods: We measured hs-cTnI (Abbott ARCHITECT) at baseline (BL) and 24 weeks (wks) in 20,718 pts enrolled in a nested biomarker study of the FOURIER trial, which tested the PCSK9i evolocumab vs. placebo in pts with ASCVD on statin and LDL >70 or non-HDL >100 mg/dL. The primary endpoint was an adjudicated composite of CV death, MI, stroke, hosp. for unstable angina, or coronary revascularization. The HR (95% CI) across percent-change of hs-cTnI was investigated in pts with quantifiable hs-cTnI at BL and 24 wks (lower limit of quantification (LLOQ) 3.6ng/L) using restricted-cubic splines and Cox-PH models, adjusted for sex, age, LDL at BL, prior MI, randomized treatment, eGFR, DM, HTN, prior HF, and BMI. A Generalized Additive Model was used to predict event rates as a function of hs-cTnIBL and absolute change, flexibly evaluated with a non-linear interaction. All models were based on 3-year follow-up using landmark analysis from 24 wks.
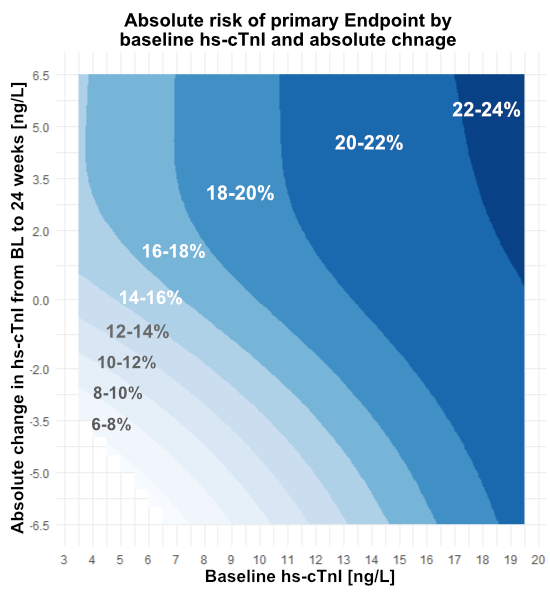
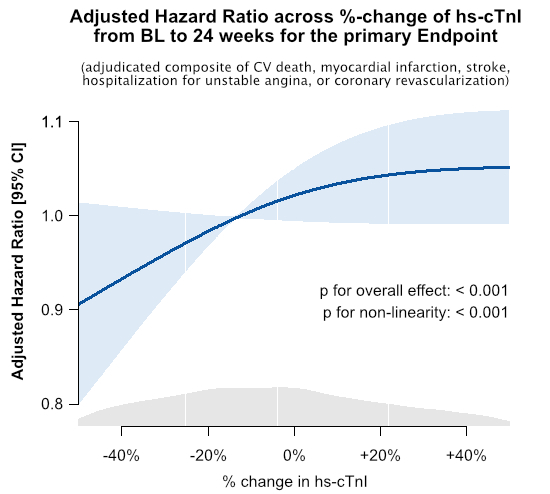
Results: Median age was 63 yrs (25th, 75th percentiles: 56, 69), 75.5% were male, 86.1% had CAD, 13.7% PAD, and 19.0% a prior stroke. Of these pts, 11,021 (46.8%) had hs-cTnI > LLOQ at BL and 24 wks. The median hs-cTnI at 24 wks was 6.6ng/L (5th, 25th, 75th, 95th percentiles are: 3.7, 4.7, 11.9, 51.1 ng/L). The absolute event rate of the primary endpoint across hs-cTnIBL and depending on absolute hs-cTnI change to 24 wks is shown in Fig. A. Higher Hs-cTnIBL was associated with higher event rates (p < 0.001). Additionally, absolute reductions and increases of hs-cTnI from BL to 24 wks resulted in reduced and increased event rates, respectively, across hs-cTnIBL. Additionally, relative decrease and increase of hs-cTnI from BL to 24 wks were associated with reduced and increased relative risk, respectively (p for overall effect < 0.001, Fig. B).
Conclusion: Changes in hs-cTnI are associated with the risk of future CV events. Serial measurements of hs-cTnI may aid in reclassification of CV risk and the identification of pts whose CV risk changes with time.
Aims: To study baseline and changes of hs-cTnI in pts with atherosclerotic CV disease (ASCVD), and the association with risk of major CV events.
Methods: We measured hs-cTnI (Abbott ARCHITECT) at baseline (BL) and 24 weeks (wks) in 20,718 pts enrolled in a nested biomarker study of the FOURIER trial, which tested the PCSK9i evolocumab vs. placebo in pts with ASCVD on statin and LDL >70 or non-HDL >100 mg/dL. The primary endpoint was an adjudicated composite of CV death, MI, stroke, hosp. for unstable angina, or coronary revascularization. The HR (95% CI) across percent-change of hs-cTnI was investigated in pts with quantifiable hs-cTnI at BL and 24 wks (lower limit of quantification (LLOQ) 3.6ng/L) using restricted-cubic splines and Cox-PH models, adjusted for sex, age, LDL at BL, prior MI, randomized treatment, eGFR, DM, HTN, prior HF, and BMI. A Generalized Additive Model was used to predict event rates as a function of hs-cTnIBL and absolute change, flexibly evaluated with a non-linear interaction. All models were based on 3-year follow-up using landmark analysis from 24 wks.
Results: Median age was 63 yrs (25th, 75th percentiles: 56, 69), 75.5% were male, 86.1% had CAD, 13.7% PAD, and 19.0% a prior stroke. Of these pts, 11,021 (46.8%) had hs-cTnI > LLOQ at BL and 24 wks. The median hs-cTnI at 24 wks was 6.6ng/L (5th, 25th, 75th, 95th percentiles are: 3.7, 4.7, 11.9, 51.1 ng/L). The absolute event rate of the primary endpoint across hs-cTnIBL and depending on absolute hs-cTnI change to 24 wks is shown in Fig. A. Higher Hs-cTnIBL was associated with higher event rates (p < 0.001). Additionally, absolute reductions and increases of hs-cTnI from BL to 24 wks resulted in reduced and increased event rates, respectively, across hs-cTnIBL. Additionally, relative decrease and increase of hs-cTnI from BL to 24 wks were associated with reduced and increased relative risk, respectively (p for overall effect < 0.001, Fig. B).
Conclusion: Changes in hs-cTnI are associated with the risk of future CV events. Serial measurements of hs-cTnI may aid in reclassification of CV risk and the identification of pts whose CV risk changes with time.
More abstracts on this topic:
Antisense Oligonucleotide Treatment of Calmodulinopathy
Bortolin Raul, Yoshinaga Daisuke, Pavlaki Nikoleta, Cavazzoni Cecilia B., T. Sage Peter, D. Whitehill Robert, Abrams Dominic, Carreon Chrystalle, Putra Juan, Alexandrescu Sanda, Guo Shuai, Nawar Farina, Tsai Owen, Rubart Michael, Kubli Dieter, Mullick Adam, Bezzerides Vassilios, Pu William, Trembley Michael, Prondzynski Maksymilian, Sweat Mason, Wang Peizhe, Chaehyoung Park, Lu Fujian, Keating Erin
Admission Acid-Base Status and Mortality in Cardiac Intensive Care Unit PatientsCanova Tyler, Lipps Kirsten, Hillerson Dustin, Kashani Kianoush, Dahiya Garima, Jentzer Jacob