Final ID: MDP1010
Galectin-3 and Progression of Kidney Disease in Patients With Type 2 Diabetes Mellitus: Analyses From the DECLARE-TIMI 58 Trial
Aims: To study the association of Gal-3 with chronic kidney disease progression, and the effect of the SGLT2-inhibitor dapagliflozin in pts with T2DM.
Methods: DECLARE-TIMI 58 was a randomized, placebo-controlled trial of dapagliflozin in pts with T2DM with or at high risk for atherosclerotic cardiovascular (CV) disease and creatinine clearance ≥60ml/min. In a nested biomarker substudy, Gal-3 was measured at baseline (Alinity, Abbott Diagnostics) in the TIMI Clinical Trials Laboratory (Boston, MA). The prespecified kidney-specific composite endpoint (Kidney-EP) was a sustained ≥40% decrease in eGFR to <60 mL/min, initiation of renal replacement therapy or confirmed sustained eGFR <15 mL/min, or adjudicated renal death. Cox models were adjusted for baseline eGFR, urine albumin-creatinine ratio (UACR), patient characteristics, CV risk factors, NTproBNP and hs-cTnT.
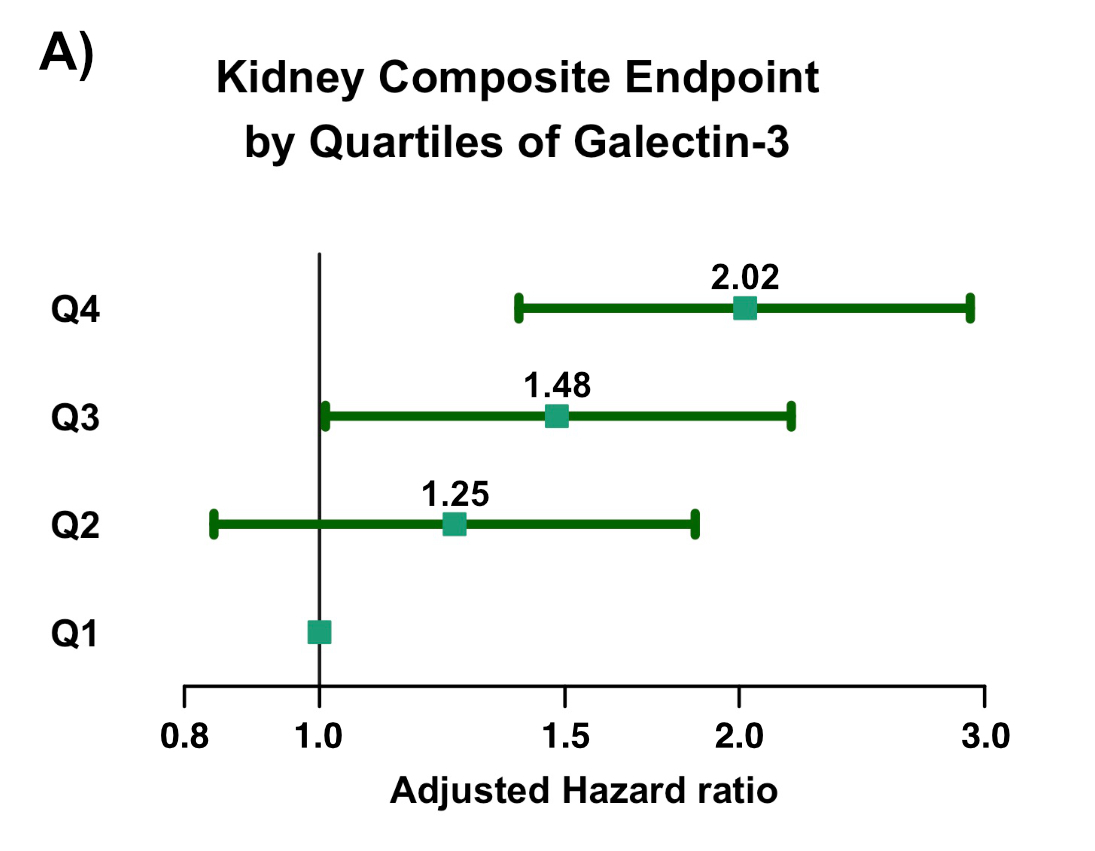
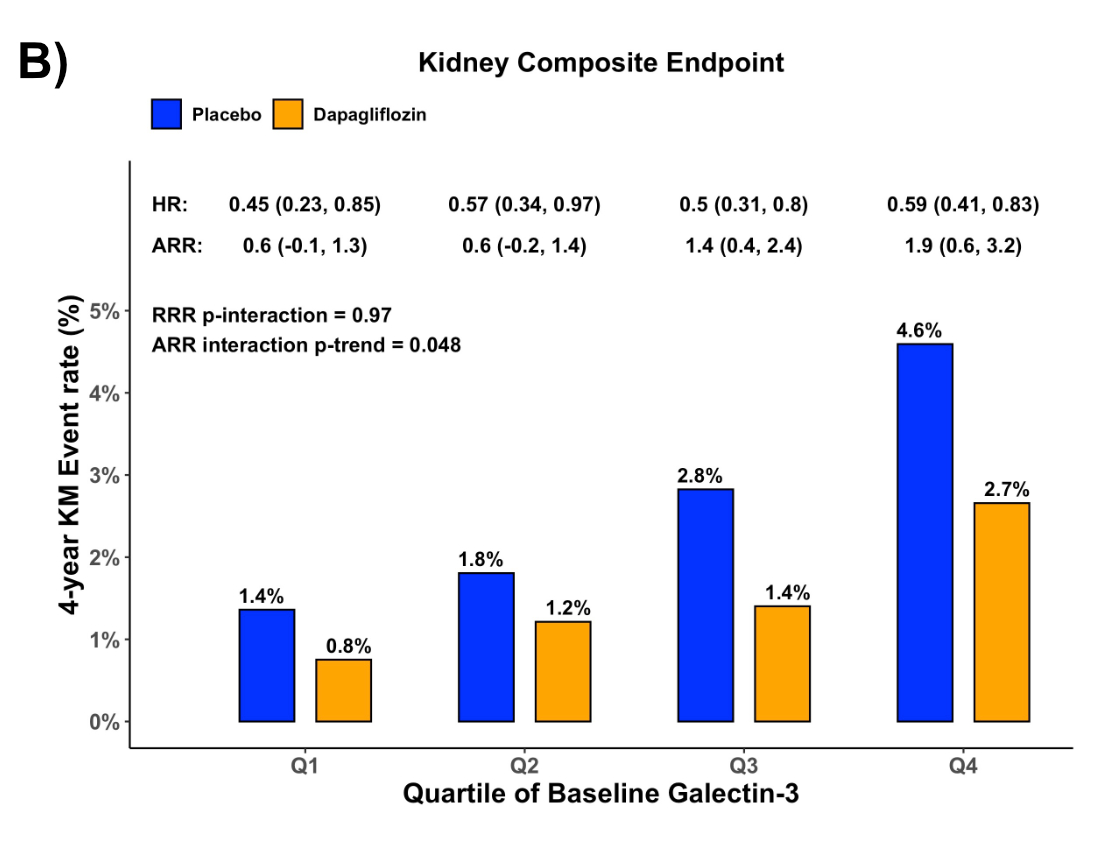
Results: Among 14,530 pts, median Gal-3 was 14.9 ng/mL [IQR, 11.9, 18.4]. Gal-3 was weakly associated with UACR (r = 0.098, p < 0.0001) and eGFR (r = -0.27, p<0.001) at baseline. Gal-3 was independently associated with the incidence of the Kidney-EP (adj-HR 1.15 [95% CI 1.03, 1.28] per 1-SD log(Gal-3), p = 0.013). Upon stratification by quartiles, there was a gradient of higher adjusted risk of the Kidney-EP with higher Gal-3 (Fig., A; p-trend <0.0001). There were 111 (1.5%) and 203 (2,8%) Kidney-EPs in the dapagliflozin and placebo groups, respectively. Dapagliflozin significantly and similarly reduced the relative risk of the Kidney-EP across quartiles of Gal-3 (overall HR 0.45 [95%CI 0.23, 0.85], p<0.0001; p-interaction = 0.97, Fig., B). However, there was greater absolute benefit of dapagliflozin with higher Gal-3 (ARR Q4 1.9 [95% CI 0.6, 3.2] vs. Q1 0.6% [-0.1, 1.3], ARR p-trend 0.048).
Conclusion: Plasma Gal-3 is associated with progression of kidney dysfunction in patients with T2DM independently of patient characteristics, including baseline kidney function. Moreover, Gal-3 identified an increasing gradient of absolute benefit of dapagliflozin for reducing kidney disease progression.
- Haller, Paul ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Mcguire, Darren ( UT Southwestern , Dallas , Texas , United States )
- Raz, Itamar ( HADASSAH HEBREW UNIVERCITY HOSPITAL , Jerusalem , Israel )
- Wilding, John ( Aintree University Hospital , Liverpool , United Kingdom )
- Sabatine, Marc ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Morrow, David ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Wiviott, Stephen ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Berg, David ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Jarolim, Petr ( BRIGHAM WOMENS HOSPITAL , Boston , Massachusetts , United States )
- Goodrich, Erica ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Bhatt, Deepak ( Icahn School of Medicine at Mount Sinai , New York City , New York , United States )
- Cahn, Avivit ( HADASSAH HEBREW UNIVERCITY HOSPITAL , Jerusalem , Israel )
- Gause-nilsson, Ingrid ( ASTRAZENECA , M Lndal , Sweden )
- Leiter, Lawrence ( St. Michael's Hospital , Toronto , Ontario , Canada )
Meeting Info:
Session Info:
The Next Stage: CKM Syndrome Progression and Implications for CVD outcomes
Sunday, 11/17/2024 , 11:10AM - 12:35PM
Moderated Digital Poster Session
More abstracts on this topic:
Gaye Ngone, Ka Mame, Kyem Damaris, Jobe Modou, Sattler Elisabeth, Gary-webb Tiffany, Gaye Bamba
Acute Effects of Isometric Handgrip Exercise on Cardiac Baroreflex Sensitivity in Chronic Kidney DiseaseSabino-carvalho Jeann, Park Jeanie
More abstracts from these authors:
Haller Paul, Giugliano Robert, O'donoghue Michelle, Scirica Benjamin, Wiviott Stephen, Braunwald Eugene, Morrow David, Ellinor Patrick, Sabatine Marc, Ruff Christian, Marston Nicholas, Melloni Giorgio, Berg David, Kamanu Frederick, Lai Yi-pin, Antman Elliott, Bhatt Deepak, Bonaca Marc, Cannon Christopher
Changes in high-sensitivity cardiac troponin I and associated cardiovascular risk: Analyses From the FOURIER TrialHaller Paul, Marston Nicholas, Bellavia Andrea, Jarolim Petr, Keech Anthony, Giugliano Robert, Sabatine Marc, Morrow David