Scientific Sessions 2024
/
Ancel Keys Memorial Lecture
/
Polygenic Risk in Heart Failure – Evaluating the Ability of a Polygenic Risk Score to Identify Risk of Future Heart Failure Events in 60,000 Patients from 6 TIMI Randomized Trials
Final ID: 4114417
Polygenic Risk in Heart Failure – Evaluating the Ability of a Polygenic Risk Score to Identify Risk of Future Heart Failure Events in 60,000 Patients from 6 TIMI Randomized Trials
Abstract Body (Do not enter title and authors here): Background: The ability of polygenic risk scores (PRS) to predict heart failure (HF) beyond classic risk factors is unclear.
Aims: To evaluate the risk of a HF PRS with incident hospitalization for HF (HHF) in patients with established cardiovascular disease.
Methods: We analyzed a 59 SNP HF PRS (HERMES collaboration, Henry et al) in genotyped patients from six multinational TIMI randomized controlled trials (DECLARE, ENGAGE AF, FOURIER, PEGASUS, SAVOR, SOLID). Patients were stratified to low (quintile, Q1), intermediate (Q2-Q4), or high (Q5) genetic risk. We investigated the association of the HF PRS with incident HHF (median of 2.5 years) i) stratified by prior HF, and ii) across NTproBNP concentrations at baseline, using Cox-proportional hazard models. Results are reported as HR [95% CI], per 1-SD or with Q1 as a reference, and adjusted for ancestry (principal components 1-5), age, sex, trial, BMI, smoking, systolic BP, prior CAD, DM, AF, and eGFR.
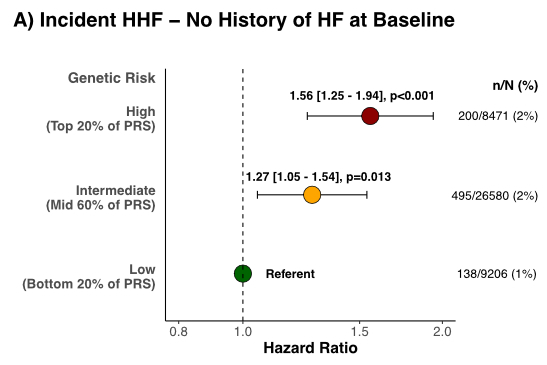
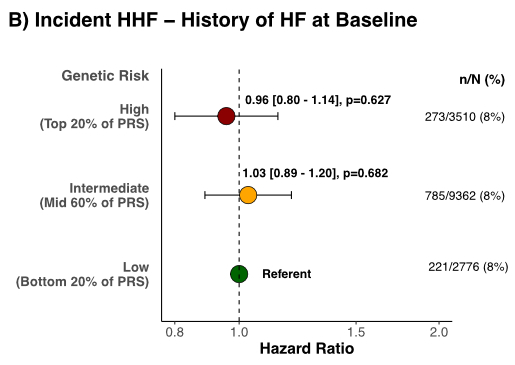
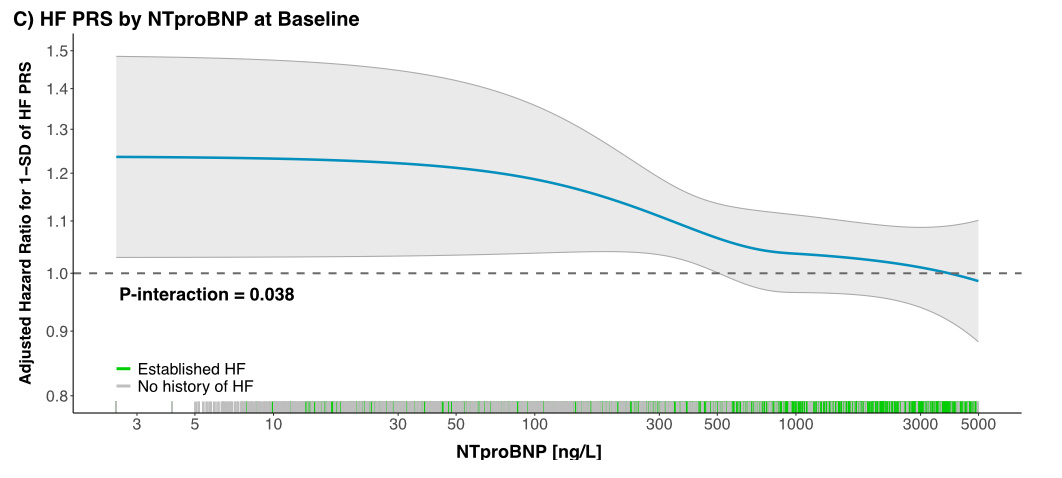
Results: In 59,906 pts (median age 66 years; 71% male; 26% prior HF), the HF PRS was associated with incident HHF (HRadj per 1-SD 1.08 [1.03, 1.13], p<0.001). Compared to low genetic risk pts, the HRadj for HHF was 1.12 [1.00, 1.26] in intermediate and 1.17 [1.02, 1.35] in high-risk pts. There were significant interactions between the HF PRS and prior HF (Pint 0.001), and NTproBNP (Pint 0.038), respectively. In pts without prior HF (Fig A), compared to low-risk pts, the risk was increased in high- (HRadj 1.56 [1.25, 1.94], p<0.001) and intermediate-risk pts (HRadj 1.27 [1.05, 1.54], p=0.013). In contrast, there was no significant association in pts with known HF at baseline (Fig B). Similarly, the HF PRS was significantly associated with HHF in pts with low but not elevated NTproBNP at baseline (Fig C).
Conclusion: Our study confirms the role of polygenic risk for HF and demonstrates that a 59 SNP PRS can identify pts at increased risk of future HF events beyond traditional clinical factors, specifically in patients without established HF or elevated NTproBNP. These findings suggest a potential application of a HF PRS for screening and early identification of pts at increased risk for HF.
Aims: To evaluate the risk of a HF PRS with incident hospitalization for HF (HHF) in patients with established cardiovascular disease.
Methods: We analyzed a 59 SNP HF PRS (HERMES collaboration, Henry et al) in genotyped patients from six multinational TIMI randomized controlled trials (DECLARE, ENGAGE AF, FOURIER, PEGASUS, SAVOR, SOLID). Patients were stratified to low (quintile, Q1), intermediate (Q2-Q4), or high (Q5) genetic risk. We investigated the association of the HF PRS with incident HHF (median of 2.5 years) i) stratified by prior HF, and ii) across NTproBNP concentrations at baseline, using Cox-proportional hazard models. Results are reported as HR [95% CI], per 1-SD or with Q1 as a reference, and adjusted for ancestry (principal components 1-5), age, sex, trial, BMI, smoking, systolic BP, prior CAD, DM, AF, and eGFR.
Results: In 59,906 pts (median age 66 years; 71% male; 26% prior HF), the HF PRS was associated with incident HHF (HRadj per 1-SD 1.08 [1.03, 1.13], p<0.001). Compared to low genetic risk pts, the HRadj for HHF was 1.12 [1.00, 1.26] in intermediate and 1.17 [1.02, 1.35] in high-risk pts. There were significant interactions between the HF PRS and prior HF (Pint 0.001), and NTproBNP (Pint 0.038), respectively. In pts without prior HF (Fig A), compared to low-risk pts, the risk was increased in high- (HRadj 1.56 [1.25, 1.94], p<0.001) and intermediate-risk pts (HRadj 1.27 [1.05, 1.54], p=0.013). In contrast, there was no significant association in pts with known HF at baseline (Fig B). Similarly, the HF PRS was significantly associated with HHF in pts with low but not elevated NTproBNP at baseline (Fig C).
Conclusion: Our study confirms the role of polygenic risk for HF and demonstrates that a 59 SNP PRS can identify pts at increased risk of future HF events beyond traditional clinical factors, specifically in patients without established HF or elevated NTproBNP. These findings suggest a potential application of a HF PRS for screening and early identification of pts at increased risk for HF.
- Haller, Paul ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Giugliano, Robert ( TIMI Study Group , Boston , Massachusetts , United States )
- O'donoghue, Michelle ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Scirica, Benjamin ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Wiviott, Stephen ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Braunwald, Eugene ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Morrow, David ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Ellinor, Patrick ( Massachusetts General Hospital , Boston , Massachusetts , United States )
- Sabatine, Marc ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Ruff, Christian ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Marston, Nicholas ( Brigham And Womens Hospital , Boston , Massachusetts , United States )
- Melloni, Giorgio ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Berg, David ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Kamanu, Frederick ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Lai, Yi-pin ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Antman, Elliott ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Bhatt, Deepak ( Icahn School of Medicine at Mount Sinai , New York City , New York , United States )
- Bonaca, Marc ( University of Colorado School of Medicine , Aurora , Colorado , United States )
- Cannon, Christopher ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
Author Disclosures:
Paul Haller: DO NOT have relevant financial relationships
| Robert Giugliano: No Answer
| Michelle O'Donoghue: DO have relevant financial relationships
;
Consultant:Janssen:Active (exists now)
; Consultant:NovoNordisk:Active (exists now)
; Consultant:Verve:Active (exists now)
; Consultant:AstraZeneca:Active (exists now)
; Consultant:Novartis:Active (exists now)
; Consultant:Amgen:Active (exists now)
; Researcher:AstraZeneca:Active (exists now)
; Researcher:Marea:Active (exists now)
; Researcher:Novartis:Active (exists now)
; Researcher:Amgen:Active (exists now)
| Benjamin Scirica: DO have relevant financial relationships
;
Research Funding (PI or named investigator):Amgen:Active (exists now)
; Individual Stocks/Stock Options:Health at Scale, Arboretum Lifesciences, and AIwithCare.com:Active (exists now)
; Consultant:Lexeo:Active (exists now)
; Consultant:Hanmi:Active (exists now)
; Consultant:Boehringer Ingelheim:Active (exists now)
; Consultant:Bayer:Active (exists now)
; Consultant:AstaZeneca:Active (exists now)
; Consultant:Amgen:Active (exists now)
; Consultant:Abbvie:Active (exists now)
; Research Funding (PI or named investigator):Verve Therapeutics:Active (exists now)
; Researcher:Pfizer:Active (exists now)
; Research Funding (PI or named investigator):Novo Nordisk:Active (exists now)
; Research Funding (PI or named investigator):Merck:Active (exists now)
; Research Funding (PI or named investigator):Milestone Pharmaceutical:Active (exists now)
; Research Funding (PI or named investigator):Boehringer Ingelheim:Active (exists now)
| Stephen Wiviott: DO have relevant financial relationships
;
Researcher:AstraZeneca:Active (exists now)
; Consultant:ICON:Active (exists now)
; Consultant:NovoNordisk:Active (exists now)
; Researcher:Merck:Active (exists now)
| Eugene Braunwald: No Answer
| David Morrow: No Answer
| Patrick Ellinor: No Answer
| Marc Sabatine: DO have relevant financial relationships
;
Research Funding (PI or named investigator):Amgen:Active (exists now)
; Research Funding (PI or named investigator):Pfizer:Active (exists now)
; Research Funding (PI or named investigator):Novartis:Active (exists now)
; Research Funding (PI or named investigator):Merck:Active (exists now)
; Research Funding (PI or named investigator):Ionis:Active (exists now)
; Consultant:AstraZeneca:Active (exists now)
; Individual Stocks/Stock Options:AstraZeneca:Active (exists now)
; Consultant:Anthos:Active (exists now)
; Research Funding (PI or named investigator):Anthos:Active (exists now)
; Consultant:Amgen:Active (exists now)
| Christian Ruff: No Answer
| Nicholas Marston: DO have relevant financial relationships
;
Speaker:Amgen:Past (completed)
; Researcher:Pfizer:Past (completed)
; Consultant:NewAmsterdam:Past (completed)
; Researcher:Marea:Active (exists now)
; Researcher:Ionis:Active (exists now)
; Researcher:Amgen:Active (exists now)
| Giorgio Melloni: DO NOT have relevant financial relationships
| David Berg: DO NOT have relevant financial relationships
| Frederick Kamanu: No Answer
| Yi-Pin Lai: No Answer
| Elliott Antman: No Answer
| Deepak Bhatt: DO have relevant financial relationships
;
Advisor:Angiowave, Bayer, Boehringer Ingelheim, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Stasys:Active (exists now)
; Other (please indicate in the box next to the company name):Trustee: American College of Cardiology; Unfunded Research: FlowCo:Active (exists now)
; Other (please indicate in the box next to the company name):Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte, Vascular Solutions;:Active (exists now)
; Royalties/Patent Beneficiary:Royalties: Elsevier (Editor, Braunwald’s Heart Disease):Active (exists now)
; Researcher:Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Alnylam, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CinCor, Cleerly, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Otsuka, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene, 89Bio;:Active (exists now)
; Royalties/Patent Beneficiary:Patent: Sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women's Hospital who assigned to Lexicon; neither I nor Brigham and Women's Hospital receive any income from this patent);:Active (exists now)
; Other (please indicate in the box next to the company name):Honoraria: Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), WebMD (CME steering committees), Wiley (steering committee);:Active (exists now)
; Other (please indicate in the box next to the company name):Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), CSL Behring (AHA lecture), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum):Active (exists now)
; Other (please indicate in the box next to the company name):Data Monitoring Committees: Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic, Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical; for ALLAY-HF, funded by Alleviant Medical), Novartis, Population Health Research Institute; Rutgers University (for the NIH-funded MINT Trial);:Active (exists now)
; Consultant:Broadview Ventures, GlaxoSmithKline, Hims, SFJ, Youngene:Active (exists now)
; Other (please indicate in the box next to the company name):Board of Directors: American Heart Association New York City, Angiowave (stock options), Bristol Myers Squibb (stock), DRS.LINQ (stock options), High Enroll (stock);:Active (exists now)
| Marc Bonaca: No Answer
| Christopher Cannon: DO have relevant financial relationships
;
Consultant:Chiesi, Amgen, Ascendia, Biogen, BI, BMS, CSL Behring, Genomadix, Lilly, Janssen, Lexicon, Milestone, Novartis, Pfizer, Rhoshan:Active (exists now)
; Research Funding (PI or named investigator):Amgen, Bayer, Cleerly, Esperion, Lexicon, Silence:Active (exists now)
; Research Funding (PI or named investigator):Amgen, Better Therapeutics, Boehringer-Ingelheim (BI), Novo Nordisk:Active (exists now)
Meeting Info:
Session Info:
More abstracts on this topic:
6-Nitrodopamine potentiates the positive chronotopic and inotropic effect induced by noradrenaline in the rat isolated heart
Lima Antonio, Sobanski Joao Fernando, Antunes Edson, De Nucci Gilberto
Changes in high-sensitivity cardiac troponin I and associated cardiovascular risk: Analyses From the FOURIER TrialHaller Paul, Marston Nicholas, Bellavia Andrea, Jarolim Petr, Keech Anthony, Giugliano Robert, Sabatine Marc, Morrow David
More abstracts from these authors:
Polygenic Risk Score Associated With Kidney Disease Progression In Patients With Cardiometabolic Risk Factors
Park Jun, Marston Nicholas, Kamanu Frederick, Melloni Giorgio, Lai Yi-pin, Giugliano Robert, Scirica Benjamin, Wiviott Stephen, Sabatine Marc, Ruff Christian
Changes in high-sensitivity cardiac troponin I and associated cardiovascular risk: Analyses From the FOURIER TrialHaller Paul, Marston Nicholas, Bellavia Andrea, Jarolim Petr, Keech Anthony, Giugliano Robert, Sabatine Marc, Morrow David
You have to be authorized to contact abstract author. Please, Login
Not Available