Final ID: Su4018
Efficacy and Safety of Empagliflozin in Patients with Acute Myocardial Infarction: A meta-analysis of randomized controlled trials
Abstract Body (Do not enter title and authors here): Background: Empagliflozin is a sodium glucose co-transporter 2 inhibitor that improves cardiovascular outcomes in patients with type 2 diabetes mellitus, chronic kidney disease and heart failure. Less is known about the efficacy and safety of empagliflozin in patients after acute myocardial infarction.
Methods: PubMed, Embase and Cochrane databases were searched for randomized controlled trials (RCTs) that reported the outcomes of interets including all-cause mortality, death from cardiovascular causes, hospitalization due to heart failure etc. Risks ratios (RR) or mean difference (MD) and their 95% confidence intervals (CIs) were computed with the use of random-effect model. Heterogeneity was examined with I2 statistics.
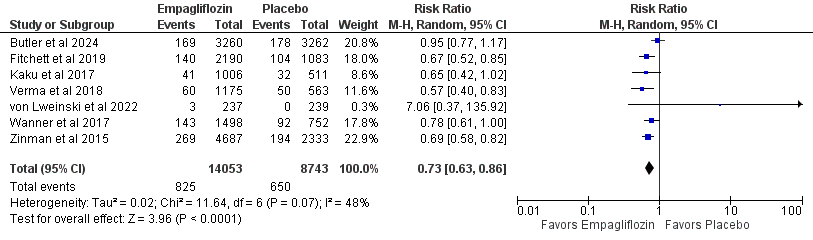
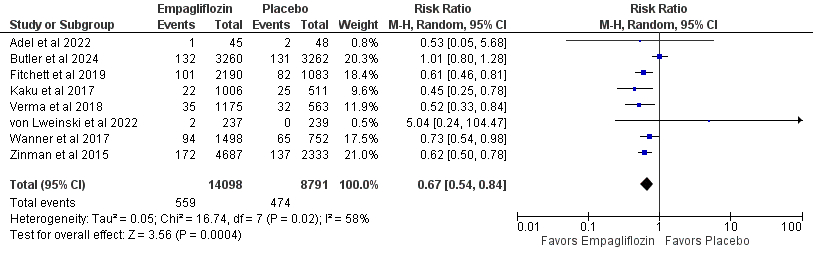
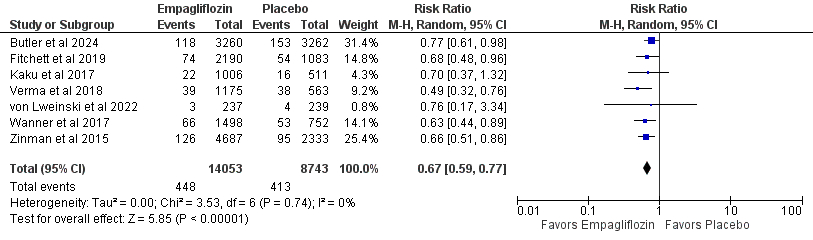
Results: We included 12 RCT articles consisting of 23,178 patients that received empagliflozin after acute myocardial infarction were considered. Empagliflozin was associated with significantly reduced incidence of all-cause mortality (RR 0.72; 95% CI [0.61, 0.85]; P < 0.0001; 5.9% vs 7.4%), death from cardiovascular causes (RR 0.66; 95% CI [0.52, 0.83]; P = 0.0004; 4.0% vs 5.4%), and hospitalization for heart failure (RR 0.66; 95% CI [0.58, 0.76]; P < 0.00001; 3.2% vs 4.7%), acute kidney injury (RR 0.52; 95% CI [0.31, 0.86]; P = 0.01), acute renal failure (RR 0.71; 95% CI [0.55, 0.91]; P = 0.008), adverse events leading to discontinuation (RR 0.89; 95% CI [0.80, 0.99]; P = 0.03), systolic blood pressure (MD -8.59; 95% CI [-13.26, -3.93], P = 0.0003), and thromboembolic events (RR 0.51; 95% CI [0.28, 0.92]; P = 0.03) compared to placebo.
However, there was no significant difference between groups with respect to fatal or nonfatal stroke (RR 1.15; 95% CI [0.91, 1.46]; P = 0.23), adverse events leading to lower limb amputation (RR 1.11; 95% CI [0.62, 1.99]; P = 0.73), estimated glomerular filtration rate (MD 0.45; 95% CI [-0.20, 1.10]; P = 0.17), diastolic blood pressure (MD -2.53; 95% CI [-5.40, 0.34]; P = 0.08), body weight (MD -1.04; 95% CI [-4.08, 1.99]; P = 0.50), urinary tract infection (MD 1.04; 95% CI [0.92, 1.17]; P = 0.55), NT- proBNP (MD -115.93; 95% CI [-271.52, 39.66]; P = 0.14), glycated hemoglobin (MD -0.10; 95% CI [-0.41, 0.22]; P = 0.55), and hypoglycemia (RR 0.98; 95% CI [0.89, 1.08]; P = 0.70).
Conclusion: Our findings suggest that empagliflozin has superior efficacy and safety compared to placebo for the treatment of patients with acute myocardial infarction with no impact on UTI, eGFR, NT-proBNP, and hypoglycemia.
Methods: PubMed, Embase and Cochrane databases were searched for randomized controlled trials (RCTs) that reported the outcomes of interets including all-cause mortality, death from cardiovascular causes, hospitalization due to heart failure etc. Risks ratios (RR) or mean difference (MD) and their 95% confidence intervals (CIs) were computed with the use of random-effect model. Heterogeneity was examined with I2 statistics.
Results: We included 12 RCT articles consisting of 23,178 patients that received empagliflozin after acute myocardial infarction were considered. Empagliflozin was associated with significantly reduced incidence of all-cause mortality (RR 0.72; 95% CI [0.61, 0.85]; P < 0.0001; 5.9% vs 7.4%), death from cardiovascular causes (RR 0.66; 95% CI [0.52, 0.83]; P = 0.0004; 4.0% vs 5.4%), and hospitalization for heart failure (RR 0.66; 95% CI [0.58, 0.76]; P < 0.00001; 3.2% vs 4.7%), acute kidney injury (RR 0.52; 95% CI [0.31, 0.86]; P = 0.01), acute renal failure (RR 0.71; 95% CI [0.55, 0.91]; P = 0.008), adverse events leading to discontinuation (RR 0.89; 95% CI [0.80, 0.99]; P = 0.03), systolic blood pressure (MD -8.59; 95% CI [-13.26, -3.93], P = 0.0003), and thromboembolic events (RR 0.51; 95% CI [0.28, 0.92]; P = 0.03) compared to placebo.
However, there was no significant difference between groups with respect to fatal or nonfatal stroke (RR 1.15; 95% CI [0.91, 1.46]; P = 0.23), adverse events leading to lower limb amputation (RR 1.11; 95% CI [0.62, 1.99]; P = 0.73), estimated glomerular filtration rate (MD 0.45; 95% CI [-0.20, 1.10]; P = 0.17), diastolic blood pressure (MD -2.53; 95% CI [-5.40, 0.34]; P = 0.08), body weight (MD -1.04; 95% CI [-4.08, 1.99]; P = 0.50), urinary tract infection (MD 1.04; 95% CI [0.92, 1.17]; P = 0.55), NT- proBNP (MD -115.93; 95% CI [-271.52, 39.66]; P = 0.14), glycated hemoglobin (MD -0.10; 95% CI [-0.41, 0.22]; P = 0.55), and hypoglycemia (RR 0.98; 95% CI [0.89, 1.08]; P = 0.70).
Conclusion: Our findings suggest that empagliflozin has superior efficacy and safety compared to placebo for the treatment of patients with acute myocardial infarction with no impact on UTI, eGFR, NT-proBNP, and hypoglycemia.
More abstracts on this topic:
A Meta-Analysis Comparing Same-Day Discharge to Later-Day Discharge in Transcatheter Aortic Valve Replacement
Jain Hritvik, Passey Siddhant, Jain Jyoti, Goyal Aman, Wasir Amanpreet, Ahmed Mushood, Patel Nandan, Yadav Ashish, Shah Janhvi, Mehta Aryan
A Focus for Improvement - Factors for Lab Adherence in a Pediatric Preventive Cardiology ProgramHolsinger Hunter, Porterfield Ronna, Taylor Makenna, Dresbach Bethany, Seipel Brittany, Igwe Chukwuemeka, Alvarado Chance, Tran Andrew