Final ID: Th0012
Development of a novel nanoparticle assay to evaluate lipoprotein bidirectional free cholesterol mass transfer
Abstract Body: Background: Free cholesterol (FC) flux between HDL and other lipoproteins or cells is a reversible process. The exchangeability of HDL-FC with peripheral tissues, particularly its direct transfer to the liver, as well as to arterial wall macrophages, affects Reverse Cholesterol Transport (RCT) and could play a major role in atherosclerosis. Thus, lipoprotein FC mass transfer may be of clinical utility in ASCVD risk assessment. Recent studies provide evidence that phospholipid-coated nanoparticles (NP) could potentially serve as a surrogate for monitoring cholesterol flux from arterial wall macrophages.
Aim: To develop an assay to measure the ability of plasma lipoproteins to either accept or donate FC.
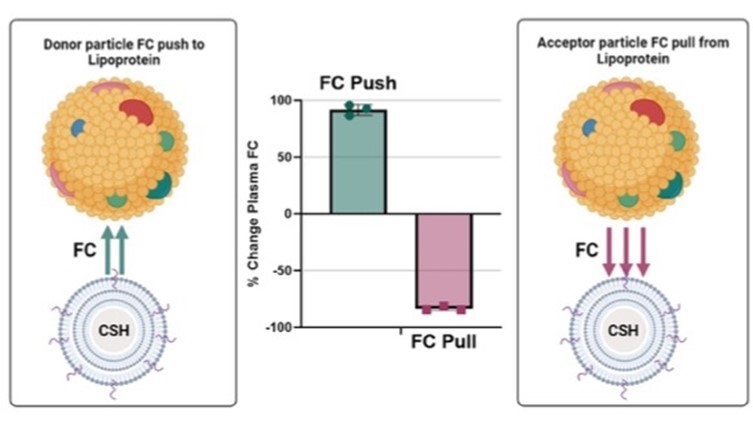
Methods/Results: We developed two novel cell-free assays to measure exchangeable pools of plasma lipoprotein FC: (i) using acceptor particles formed by coating calcium silicate hydrate (CSH) nanoparticles with excess phospholipid (FC “Pull”) and (ii) delivery of additional FC to HDL and other lipoproteins using same nanoparticles pre-saturated with FC (FC “Push”). We optimized the phospholipid mass required to fully coat CSH particles and the excess FC required for FC push. FC mass transferred from human plasma (HP) to acceptor particles varied by sample (≈20-40%) and by lipoprotein type (LDL; 70%, HDL; 47%). FC removal was time- and temperature-dependent. Kinetic studies suggest that the FC transfer rate was initially rapid (0-20 min) and slowly decreased thereafter (40-60 min). Similarly, HP FC uptake from FC donor particles also varied between samples and was time- and temperature dependent. FC uptake was exclusively observed in HDL. Following ApoB depletion (AD), the level of FC uptake by HDL remained unchanged but represented 50% of the FC removal in HP. These findings were replicated by lipid analysis of lipoproteins isolated by fast performance liquid chromatography (FPLC). LDL and HDL both showed significant removal of FC but FC uptake only occurred with HDL. We then validated our results in HDL receptor deficient mice (Scarb1-/-), which have elevated HDL enriched in FC. Scarb1-/- mice did not present any FC uptake, whereas WT mice had a significant uptake. However, Scarb1-/- were more potent donor of FC (≈2.5×) than WT mice.
Conclusions: We have developed a novel cell-free assays to measure FC mass transfer to and from plasma lipoproteins. Future studies will examine if the FC push/pull ratio may serve as a useful metric for ASCVD risk.
Aim: To develop an assay to measure the ability of plasma lipoproteins to either accept or donate FC.
Methods/Results: We developed two novel cell-free assays to measure exchangeable pools of plasma lipoprotein FC: (i) using acceptor particles formed by coating calcium silicate hydrate (CSH) nanoparticles with excess phospholipid (FC “Pull”) and (ii) delivery of additional FC to HDL and other lipoproteins using same nanoparticles pre-saturated with FC (FC “Push”). We optimized the phospholipid mass required to fully coat CSH particles and the excess FC required for FC push. FC mass transferred from human plasma (HP) to acceptor particles varied by sample (≈20-40%) and by lipoprotein type (LDL; 70%, HDL; 47%). FC removal was time- and temperature-dependent. Kinetic studies suggest that the FC transfer rate was initially rapid (0-20 min) and slowly decreased thereafter (40-60 min). Similarly, HP FC uptake from FC donor particles also varied between samples and was time- and temperature dependent. FC uptake was exclusively observed in HDL. Following ApoB depletion (AD), the level of FC uptake by HDL remained unchanged but represented 50% of the FC removal in HP. These findings were replicated by lipid analysis of lipoproteins isolated by fast performance liquid chromatography (FPLC). LDL and HDL both showed significant removal of FC but FC uptake only occurred with HDL. We then validated our results in HDL receptor deficient mice (Scarb1-/-), which have elevated HDL enriched in FC. Scarb1-/- mice did not present any FC uptake, whereas WT mice had a significant uptake. However, Scarb1-/- were more potent donor of FC (≈2.5×) than WT mice.
Conclusions: We have developed a novel cell-free assays to measure FC mass transfer to and from plasma lipoproteins. Future studies will examine if the FC push/pull ratio may serve as a useful metric for ASCVD risk.
More abstracts on this topic:
An Economic Evaluation of Non-HDL-Cholesterol and Apolipoprotein B as Treatment Targets for Lipid-Lowering Therapy in Primary Prevention
Luebbe Samuel, Wilkins John, Moran Andrew, Sniderman Allan, Kohli-lynch Ciaran
Accelerated Biological Aging, Early-Life Exposure to Tobacco, and Incident Aortic Aneurysm: A Large-Scale Prospective Cohort Study in UK BiobankYang Miaomiao, Feng Weijing, Dang Aimin, Gu Yingzhen