Final ID: TP211
In silico drug repurposing identifies transcriptional mitigators of blood-induced neurotoxicity in an organoid model of intracerebral hemorrhage
Abstract Body: Introduction:
The optimal therapeutic window for evacuation of intracerebral hemorrhage (ICH) is controversial, balancing risk of prolonged exposure to blood-induced neurotoxicity against risk of post-operative rebleeding. Pharmacologic mitigators of neurotoxicity have the potential to lengthen this therapeutic window, extending the opportunity for favorable surgical outcomes. Here, we identify a blood-induced transcriptomic signature in an organoid model of ICH and perform an in silico screen to identify reversers of blood-induced toxicity.
Methods:
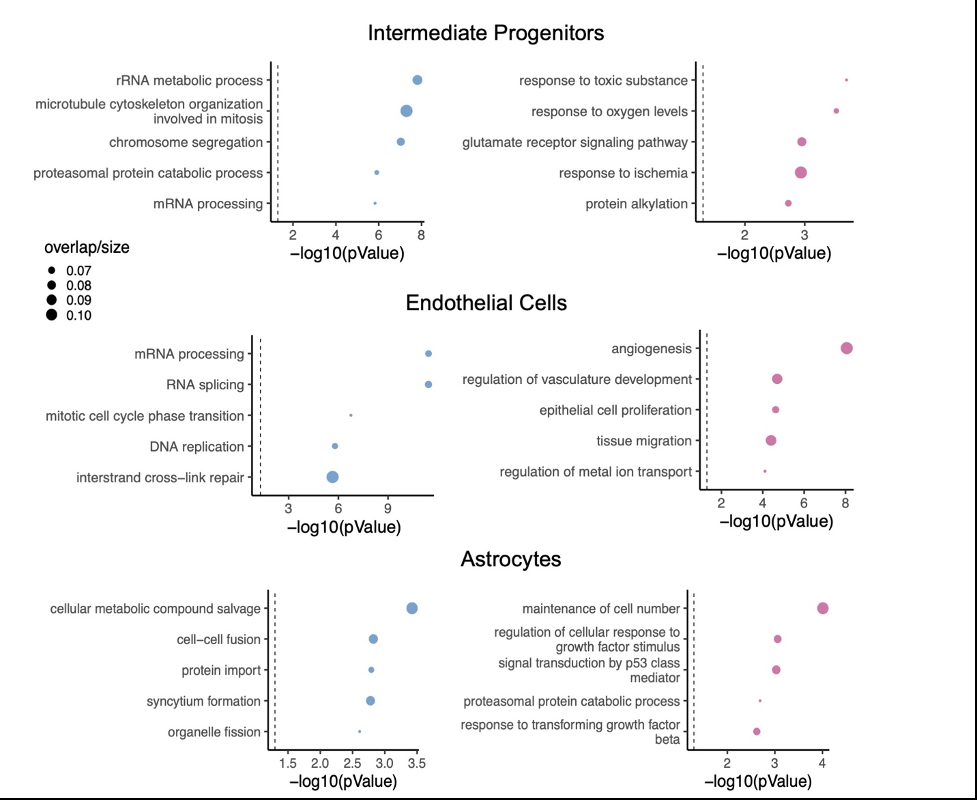
Single-cell RNA sequencing was performed on 96,725 cells across 18 human neural organoids comprising multiple cell types, treated with 5% blood for either 6 or 24 hours. Alignment, filtering, and analysis was performed using cellranger, SoupX, Scrublet, and Seurat. Gene associations to blood exposure were conducted using mixed-effect linear modeling. Significant expression signatures were determined using Benjamini-Hochberg multiple testing correction (FDR <5%). To identify compounds that may reverse the blood-exposure signature, we applied the Broad Institute Connectivity Map query tool to calculate a weighted enrichment score between the blood-induced signature and curated expression signatures of known therapeutic compounds. Compounds with a significant enrichment score FDR<0.05 were considered “reversers”.
Results:
Single-cell sequencing identified distinct transcriptomic impacts of 24-hours of blood exposure, including astrocyte reactivity (TGFb response, p=2.41x10-3), endothelial vasculogenesis (p=8.73x10-9), and neurotoxicity (glutamate receptor signaling, p= 1.12x10-3). To identify therapeutics with potential to reverse this signature, we performed an in silico drug screen, identifying 345 compounds targeting 119 unique mechanisms of action. Ten classes of drugs reversed blood-induced signatures in all three cell types, including adrenergic receptor antagonists, dopamine receptor antagonists, histamine receptor antagonists, acetylcholinesterase inhibitors, calcium channel blockers, phosphodiesterase inhibitors, HDAC inhibitors, MAPK inhibitors, and antibiotics.
Conclusion:
The neurotoxic effects of blood may be mitigated by a number of compounds targeting convergent mechanisms of toxicity across cell types. These candidates may represent novel therapeutic compounds to widen the window for safe and effective ICH evacuation and are ideal candidates for screening in scalable in vitro organoid models of ICH.
The optimal therapeutic window for evacuation of intracerebral hemorrhage (ICH) is controversial, balancing risk of prolonged exposure to blood-induced neurotoxicity against risk of post-operative rebleeding. Pharmacologic mitigators of neurotoxicity have the potential to lengthen this therapeutic window, extending the opportunity for favorable surgical outcomes. Here, we identify a blood-induced transcriptomic signature in an organoid model of ICH and perform an in silico screen to identify reversers of blood-induced toxicity.
Methods:
Single-cell RNA sequencing was performed on 96,725 cells across 18 human neural organoids comprising multiple cell types, treated with 5% blood for either 6 or 24 hours. Alignment, filtering, and analysis was performed using cellranger, SoupX, Scrublet, and Seurat. Gene associations to blood exposure were conducted using mixed-effect linear modeling. Significant expression signatures were determined using Benjamini-Hochberg multiple testing correction (FDR <5%). To identify compounds that may reverse the blood-exposure signature, we applied the Broad Institute Connectivity Map query tool to calculate a weighted enrichment score between the blood-induced signature and curated expression signatures of known therapeutic compounds. Compounds with a significant enrichment score FDR<0.05 were considered “reversers”.
Results:
Single-cell sequencing identified distinct transcriptomic impacts of 24-hours of blood exposure, including astrocyte reactivity (TGFb response, p=2.41x10-3), endothelial vasculogenesis (p=8.73x10-9), and neurotoxicity (glutamate receptor signaling, p= 1.12x10-3). To identify therapeutics with potential to reverse this signature, we performed an in silico drug screen, identifying 345 compounds targeting 119 unique mechanisms of action. Ten classes of drugs reversed blood-induced signatures in all three cell types, including adrenergic receptor antagonists, dopamine receptor antagonists, histamine receptor antagonists, acetylcholinesterase inhibitors, calcium channel blockers, phosphodiesterase inhibitors, HDAC inhibitors, MAPK inhibitors, and antibiotics.
Conclusion:
The neurotoxic effects of blood may be mitigated by a number of compounds targeting convergent mechanisms of toxicity across cell types. These candidates may represent novel therapeutic compounds to widen the window for safe and effective ICH evacuation and are ideal candidates for screening in scalable in vitro organoid models of ICH.
More abstracts on this topic:
Early Intensive Blood Pressure Lowering within 6 hours of Intracerebral Hemorrhage Onset is Associated with Improved Functional Outcomes in Larger Trials, but Inconclusive Evidence Overall: A Meta-Analysis with Trial Sequential Analysis of Randomized Controlled Trials
Rajith Gokul, Erzinger Gabriel, Rizwan Ahmed Aisha, Silva Yasmin, Noll Giovani, Hussain Masaraf, Premraj Lavienraj
A Randomized, Double Blinded, Phase 2B Clinical Trial to Compare the Safety and Efficacy of Sodium Chloride and Sodium Acetate Combination Intravenous Fluids in Acute Stroke PatientsWasay Muhammad, Suri Fareed, Saleem Shafaq, Qureshi Adnan
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.
Rate this abstract
(Maximum characters: 500)