Final ID: WP176
Patients’ and Surrogates’ Perspectives on Consent for an Acute Stroke Clinical Trial
Abstract Body: Introduction: Informed consent for clinical trials in the acute stroke setting is challenging. There is a need for context-appropriate approaches to consent, but few data exist regarding implementation of innovative approaches. In the Multi-Arm Optimization of Stroke Thrombolysis (MOST) trial (NCT03735979), a consent process was designed in collaboration with patient advisors that included a short consent form and a companion information sheet. This approach was implemented at all study sites, and participants’ experiences were assessed using a post-enrollment survey.
Methods: All participants enrolled in MOST were eligible for the survey. The person who provided consent for enrollment (patient or surrogate) was asked to fill out the survey. The survey was adapted from a prior survey of patients’ and surrogates’ experiences with consent in acute care research and was cognitively pre-tested. Descriptive statistics were tabulated. Likert scale responses on a scale of 1-5 with 1 being strongly agree and 5 being strongly disagree and on a scale of 1-5 with 1 being extremely helpful and 5 being not helpful at all were collapsed into agree (1-2)/not agree (3-5) and helpful (1-2)/not helpful (3-5), respectively.
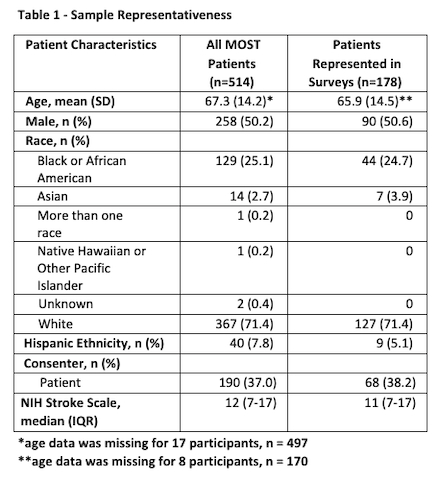
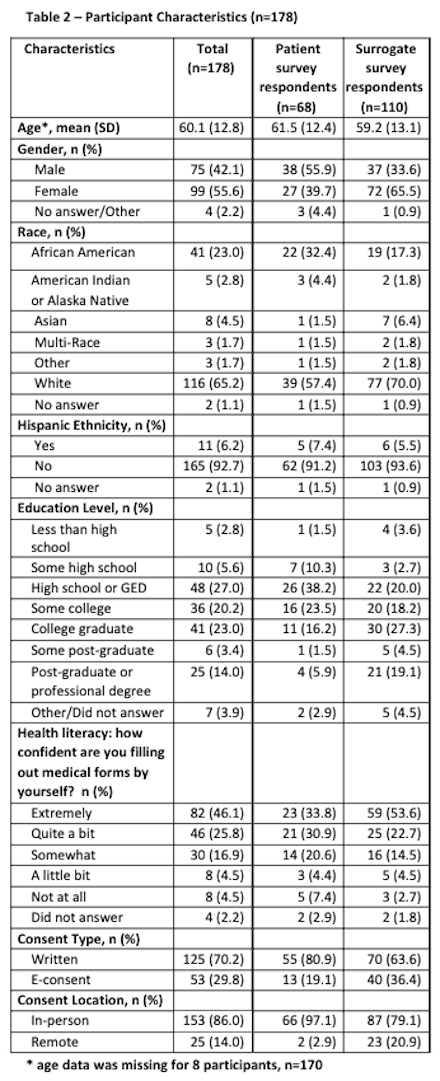
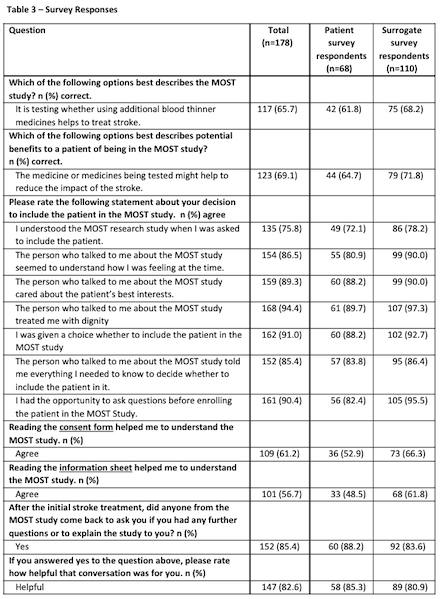
Results: There were 195 completed surveys out of 514 enrollments in the MOST trial (overall capture rate 37.9%). Seventeen surveys were excluded due to mismatch between who consented to MOST and who completed the survey (total n=178 analyzable surveys). Patients completing the survey (or for whom a surrogate completed the survey) were similar to the overall enrolled population in terms of age, sex, race, and stroke severity (Table 1). The average age of survey respondents was 60.1 years, with 42.1% being male and 61.8% being surrogates (Table 2). Overall patients’ and surrogates’ experiences were positive. Post-enrollment communication and consent materials were viewed favorably (Table 3). Open-ended feedback was positive; participants acknowledged that time stress was intrinsic to the situation, encouraged simplicity, and offered few suggestions for improvement.
Conclusions: A patient-centered consent process in an acute stroke trial was positively viewed by both patients and surrogates. Embedding assessments of patients’ and surrogates’ experiences within clinical trials offers an important opportunity for understanding the impact of innovation regarding consent.
Methods: All participants enrolled in MOST were eligible for the survey. The person who provided consent for enrollment (patient or surrogate) was asked to fill out the survey. The survey was adapted from a prior survey of patients’ and surrogates’ experiences with consent in acute care research and was cognitively pre-tested. Descriptive statistics were tabulated. Likert scale responses on a scale of 1-5 with 1 being strongly agree and 5 being strongly disagree and on a scale of 1-5 with 1 being extremely helpful and 5 being not helpful at all were collapsed into agree (1-2)/not agree (3-5) and helpful (1-2)/not helpful (3-5), respectively.
Results: There were 195 completed surveys out of 514 enrollments in the MOST trial (overall capture rate 37.9%). Seventeen surveys were excluded due to mismatch between who consented to MOST and who completed the survey (total n=178 analyzable surveys). Patients completing the survey (or for whom a surrogate completed the survey) were similar to the overall enrolled population in terms of age, sex, race, and stroke severity (Table 1). The average age of survey respondents was 60.1 years, with 42.1% being male and 61.8% being surrogates (Table 2). Overall patients’ and surrogates’ experiences were positive. Post-enrollment communication and consent materials were viewed favorably (Table 3). Open-ended feedback was positive; participants acknowledged that time stress was intrinsic to the situation, encouraged simplicity, and offered few suggestions for improvement.
Conclusions: A patient-centered consent process in an acute stroke trial was positively viewed by both patients and surrogates. Embedding assessments of patients’ and surrogates’ experiences within clinical trials offers an important opportunity for understanding the impact of innovation regarding consent.
More abstracts on this topic:
Adults with Arrhythmias and Cardiomyopathy Have Lower WHO-QOL Health Satisfaction and Different Wellness Intervention Preferences
Sandau Kristin, Mathiason Michelle, Bai Ling, Conley Samantha
Assessing Social Vulnerability's Effect on Patient Outcomes in Los Angeles County Stroke Patients Among a Health System and Its Impact on Health DisparitiesMayorga Lina, Bohn Joe, Levin Dr Bruce L, Kim-tenser May, Sanossian Nerses, Towfighi Amytis
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.
Rate this abstract
(Maximum characters: 500)