Final ID: 15
Integrated Genomic and Proteomic Drug Target Discovery for Ischemic Stroke
Abstract Body: Background: Ischemic stroke (IS) is a multifactorial disease with a significant genetic component contributing about 40% of the risk. Current prevention strategies focus on risk factor control, but integrating genomic and proteomic data could uncover key molecular targets for more effective treatments based on genetic insights.
Objective: To utilize a comprehensive multi-omic approach to identify novel drug targets that mediate the genetic risk of IS.
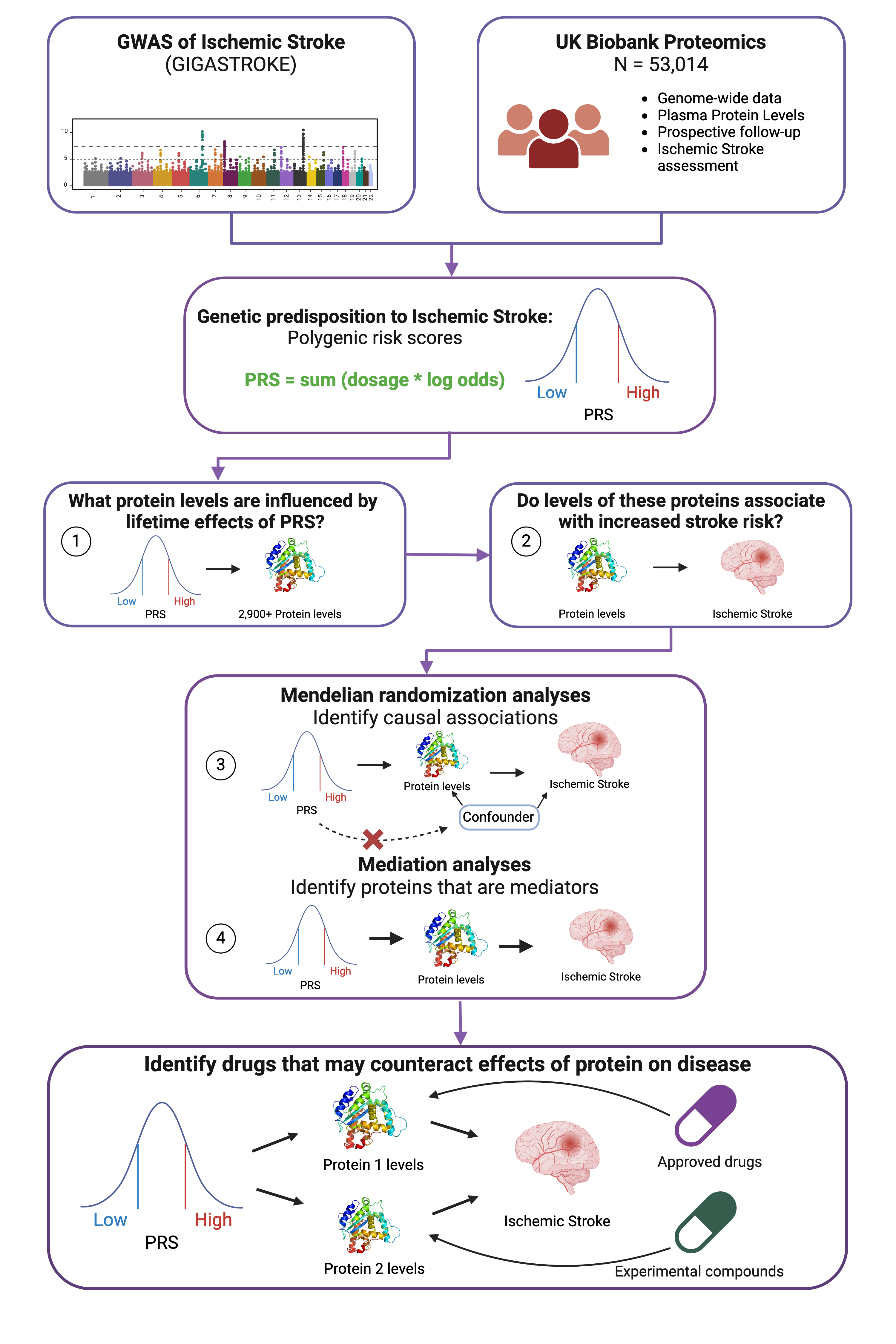
Methods: We analyzed genomic and proteomic data from 53,014 UK Biobank participants. Using a polygenic risk score (PRS) for IS from 43 independent risk variants, we deployed four analytical steps (Fig. 1, all steps corrected for multiple testing): (1) linear regression between PRS and 2,923 standardized protein levels measured at baseline, adjusted for age, sex, and genetic principal components; (2) association between selected proteins and IS; (3) Mendelian Randomization to assess causality for the proteins from (1+2), and (4) mediation to quantify the intermediary role of causal proteins in the PRS-stroke relationship. To provide clinical context, we conducted a proof-of-concept analysis using Alzheimer's disease (AD) given APOE's established role in AD risk.
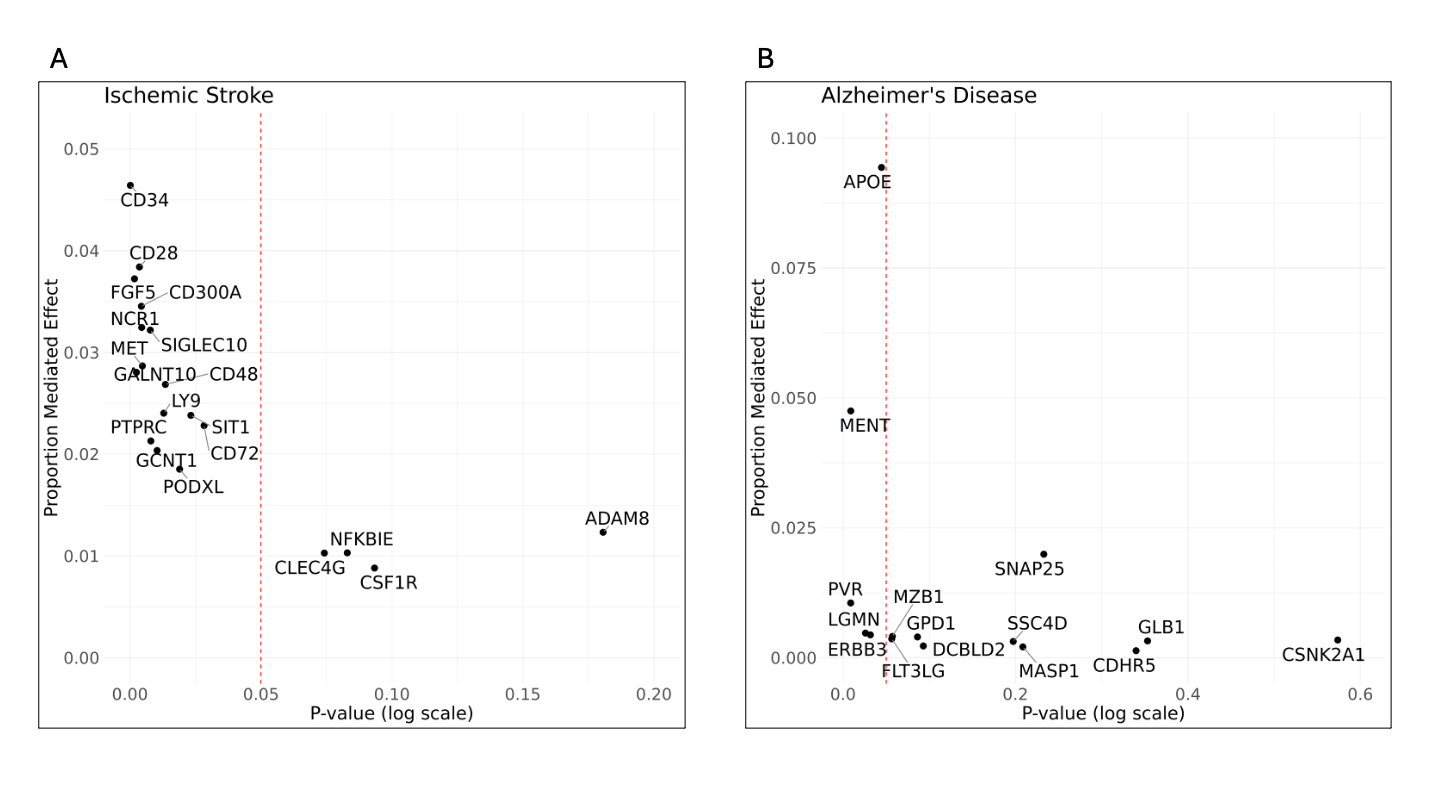
Results: We found 15 proteins that causally mediate the association between polygenic risk and IS (Fig. 2). The identified proteins are involved in cell adhesion (e.g. CD34, CD48, PODXL), inflammatory pathways (e.g. CD28, CD300A, NCR1), angiogenesis (FGF5, MET), and protein glycosylation (GALNT10, GCNT1), influencing vascular integrity, immune response, and blood vessel formation. Mediated effects range from 1-5% per protein and two proteins served as targets for existing or developing drugs (Tab. 1). As proof-of-concept, we discovered five proteins with significant mediation in AD, the strongest effect seen in APOE (10% mediated effect) and MENT (5%), confirming their known roles in neurodegenerative processes.
Conclusion: This multi-omic strategy successfully identified 15 proteins mediating polygenic risk of IS, highlighting crucial pathways such as cell adhesion, inflammation, angiogenesis, and glycosylation. These findings can advance targeted therapies for stroke risk in primary prevention. Our proof-of-concept study illustrates the clinical meaning of the proteins in comparison with the known impact of APOE on AD. Future work should focus on validating these targets in clinical settings and exploring drug repurposing opportunities.
Objective: To utilize a comprehensive multi-omic approach to identify novel drug targets that mediate the genetic risk of IS.
Methods: We analyzed genomic and proteomic data from 53,014 UK Biobank participants. Using a polygenic risk score (PRS) for IS from 43 independent risk variants, we deployed four analytical steps (Fig. 1, all steps corrected for multiple testing): (1) linear regression between PRS and 2,923 standardized protein levels measured at baseline, adjusted for age, sex, and genetic principal components; (2) association between selected proteins and IS; (3) Mendelian Randomization to assess causality for the proteins from (1+2), and (4) mediation to quantify the intermediary role of causal proteins in the PRS-stroke relationship. To provide clinical context, we conducted a proof-of-concept analysis using Alzheimer's disease (AD) given APOE's established role in AD risk.
Results: We found 15 proteins that causally mediate the association between polygenic risk and IS (Fig. 2). The identified proteins are involved in cell adhesion (e.g. CD34, CD48, PODXL), inflammatory pathways (e.g. CD28, CD300A, NCR1), angiogenesis (FGF5, MET), and protein glycosylation (GALNT10, GCNT1), influencing vascular integrity, immune response, and blood vessel formation. Mediated effects range from 1-5% per protein and two proteins served as targets for existing or developing drugs (Tab. 1). As proof-of-concept, we discovered five proteins with significant mediation in AD, the strongest effect seen in APOE (10% mediated effect) and MENT (5%), confirming their known roles in neurodegenerative processes.
Conclusion: This multi-omic strategy successfully identified 15 proteins mediating polygenic risk of IS, highlighting crucial pathways such as cell adhesion, inflammation, angiogenesis, and glycosylation. These findings can advance targeted therapies for stroke risk in primary prevention. Our proof-of-concept study illustrates the clinical meaning of the proteins in comparison with the known impact of APOE on AD. Future work should focus on validating these targets in clinical settings and exploring drug repurposing opportunities.
More abstracts on this topic:
β1 Adrenergic Receptor Autoantibodies Promote Heart Failure Though Activation of Prostaglandin E2 Receptor EP1/Phosphodiesterase 4B Pathway
Cao Ning, Qiu Hui, Li Hongwei
A Multicenter, Prospective, Randomized Controlled Trial of Endovascular Treatment with or without Intravenous ThromBolysis in Acute Ischemic Stroke of Basilar Artery Occlusion (BEST-BAO): Study ProtocolXiang Yang, Siddiqui Adnan, Yang Shu, Mocco J, Yu Nengwei, Schonewille Wouter, Guo Fuqiang
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.
Rate this abstract
(Maximum characters: 500)