Final ID: 141
Clinical Trial Emulation Leveraging Genetic Effects: A Proof-of-Concept Application to SPRINT
Methods: We conducted SPRINT-e, a genomic emulation of the SPRINT RCT, using UK Biobank data. SPRINT originally randomized 9,361 hypertensive individuals without diabetes into two groups: intensive treatment (SBP<120 mmHg, n=4,678) and standard treatment (SBP<140 mmHg, n=4,683). We selected participants meeting SPRINT’s criteria and used a validated polygenic risk score for SBP to emulate treatment effects. Starting with the 4,678 participants with the lowest genetic risk, we iteratively replaced low-risk participants with higher-risk ones until we matched SPRINT’s SBP difference (15 mmHg) and standard treatment arm size. Finally, we used Cox models, as in SPRINT, to assess whether intensive treatment reduced the risk of stroke, myocardial infarction, coronary artery disease, and cardiovascular death.
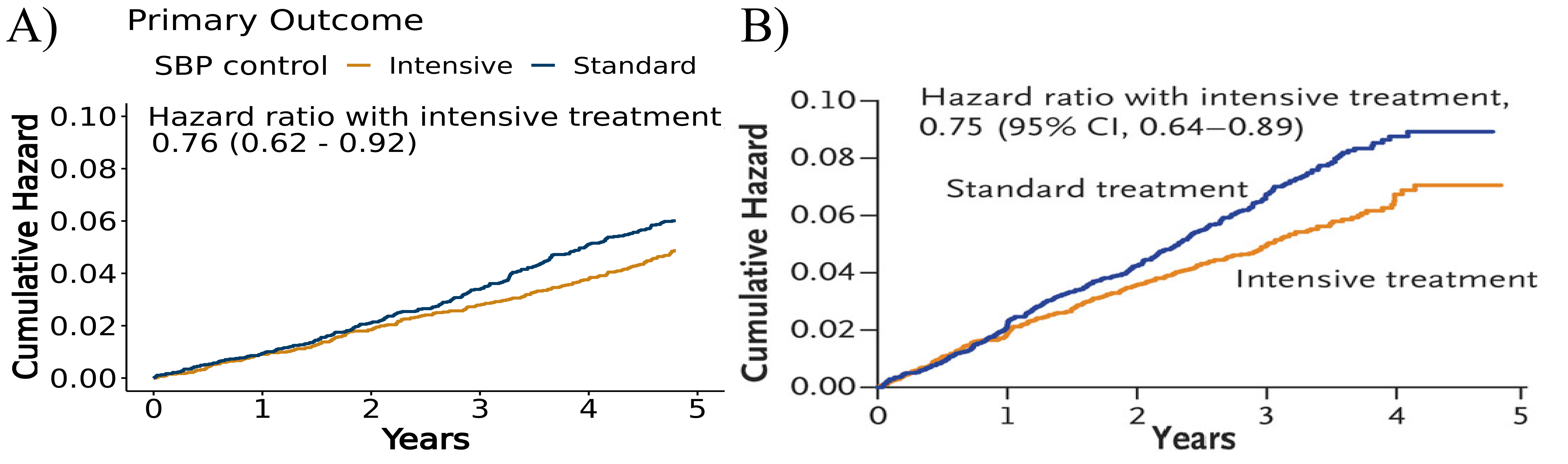
Results: Like SPRINT, SPRINT-e included 4678 participants assigned to intensive genetic treatment, 4683 participants assigned to standard genetic treatment (both mean age 63, 42% female), and a follow-up time of 4.8 years. The number of events in the intensive treatment group of SPRINT and SPRINT-e was 243(5.2%) and 220(4.7%), respectively, while in the standard treatment groups was 319(6.8%) and 270(5.8%), respectively. In SPRINT and SPRINT-e, Cox proportional hazard models estimated that intensive versus standard treatment led to risk reductions in the composite outcome of 35%(HR: 0.75, 95%CI: 0.64-0.89) and 34%(HR: 0.76, 95%CI: 0.62–0.92), respectively (Figure 1).
Conclusion: Our genomic-based RCT emulation framework accurately reproduced the primary result of SPRINT. Although the absolute event rate in SPRINT-e was lower than in SPRINT, the relative risk reductions were nearly identical. This framework could be valuable for simulating RCTs that target continuous and highly heritable physiological variables. Utilizing these genomic tools could significantly improve the success rate of future RCTs.
More abstracts on this topic:
Bulut Aybike, Karacan Mehmet, Saygili E. Alper, Pirli Dogukan, Aydin Eylul, Ozdemir Ozkan, Balci Nermin, Alanay Yasemin, Bilguvar Kaya, Akgun Dogan Ozlem
10-Year Trend Analysis of Medicare Payment in Stroke Inpatient Hospital AdmissionWong Ka-ho, Krothapalli Neeharika, Littig Lauren, Champagne Alison, Majersik Jennifer, Reddy Vivek, De Havenon Adam
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.