Final ID: 86
Location-Specific Hematoma Volume Tolerance for Spontaneous Intracerebral Hemorrhage
Hematoma volume is an important predictor of outcome in spontaneous intracerebral hemorrhage (ICH). Location-specific hematoma volume thresholds are associated with poor outcome and can inform surgical trial inclusion criteria and clinical decision rules for hematoma evacuation. In a pooled trial dataset, we evaluated associations between ICH location, hematoma volume thresholds, and patient outcomes.
Methods
We performed a secondary analysis of the ATACH-2 clinical trial. We evaluated the associations between intraparenchymal location-specific hematoma volume cutoffs (thalamic, basal ganglia, and lobar) and poor outcome (mRS 4-6; primary outcome) or mortality (secondary outcome) at 3 months. Using semi-automated volumetric assessments of 24-hour CT scans, we applied volume cutoffs at 5 mL increments starting at ≤5 mL up to >50 mL. We also applied Youden’s method for each hematoma location to determine the optimal location-specific volume thresholds that predict outcomes. We calculated odds ratios (OR) of poor outcome through multivariable logistic regression models for each location, adjusted for age, sex, prior stroke/transient ischemic attack, hemisphere location, and intraventricular hemorrhage extension.
Results
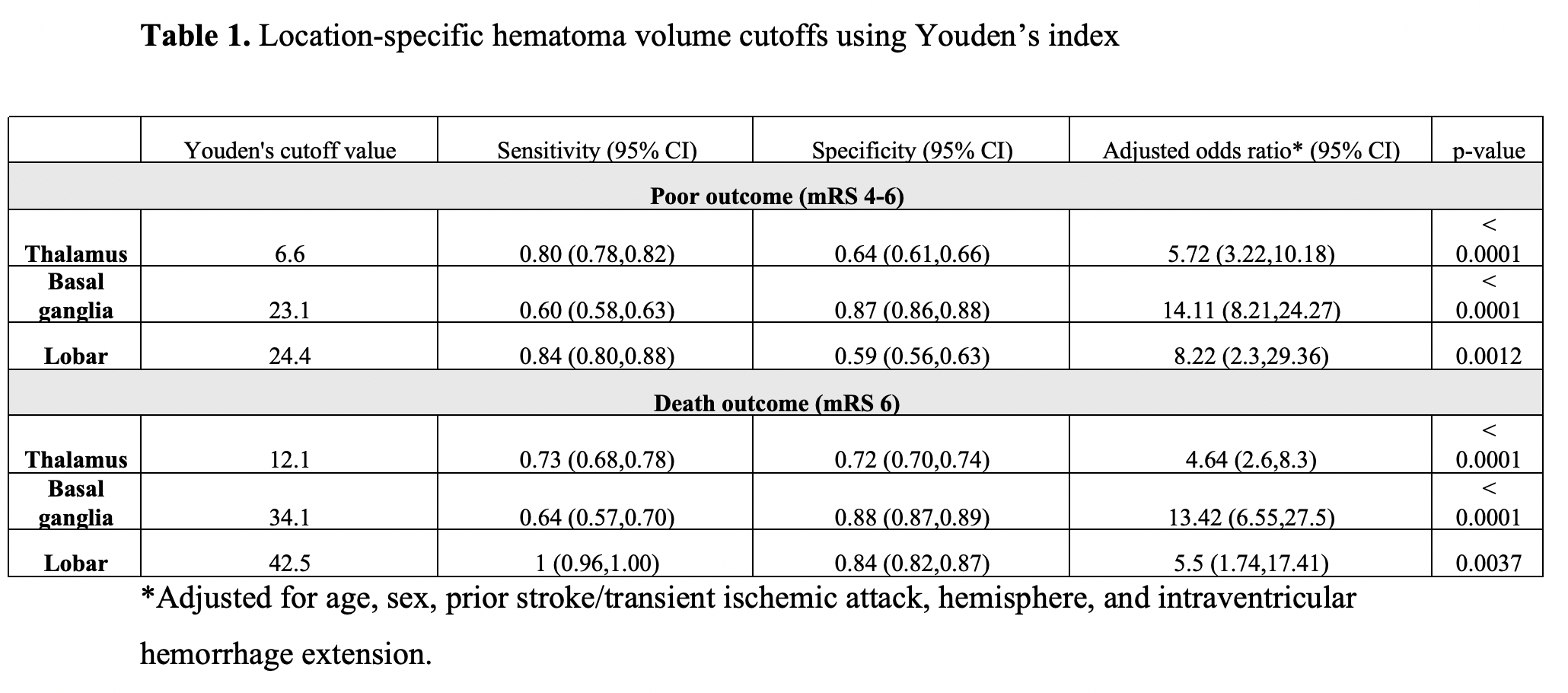
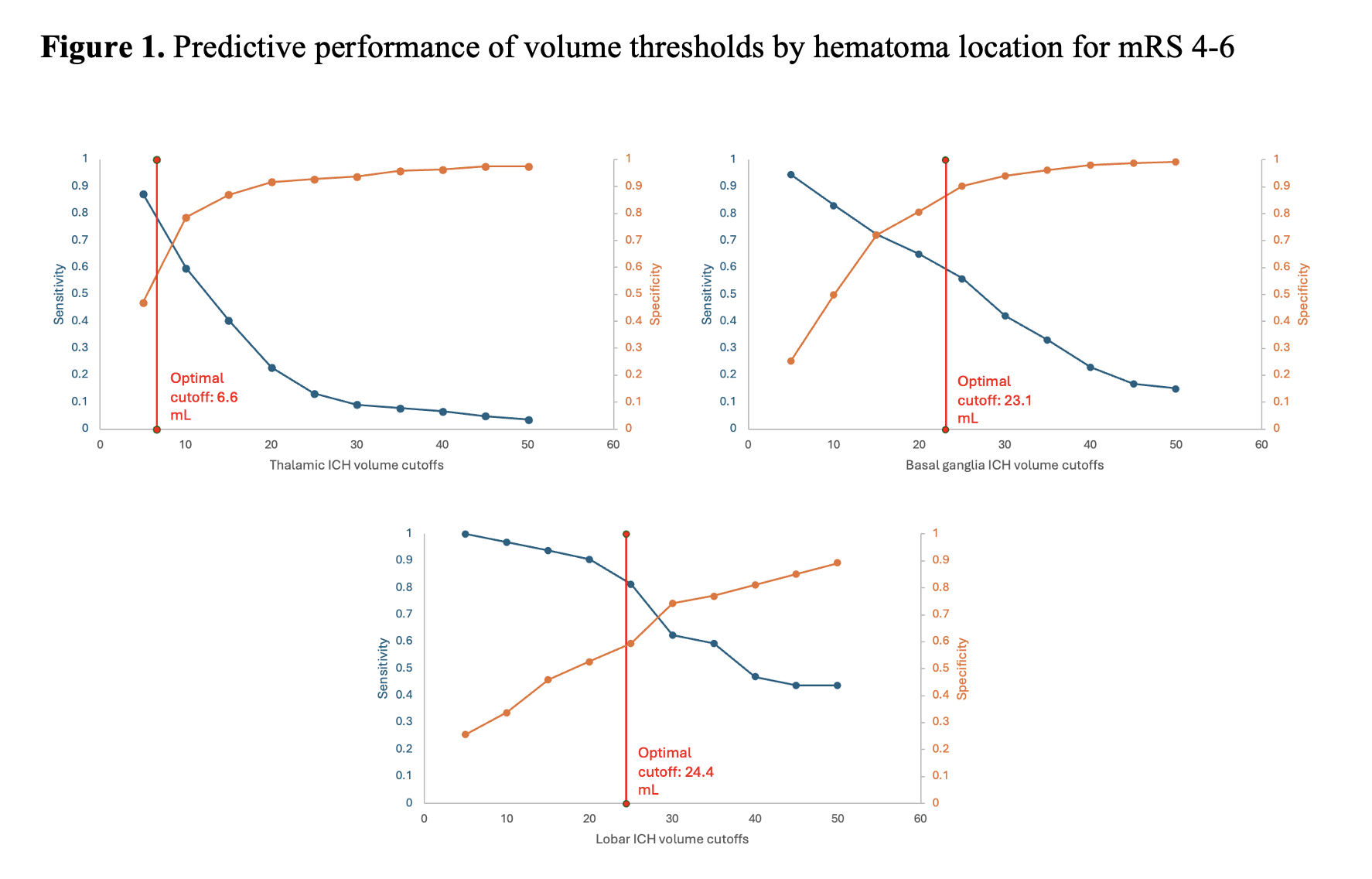
Out of 949 patients included for analysis, 358, 485 and 106 were diagnosed with thalamic, basal ganglia ICH and lobar ICH, respectively. Location-specific hematoma volume cutoffs most predictive of a poor outcome (mRS 4-6) calculated with Youden’s index were 6.6 mL for thalamic ICH (OR 5.72, 95% CI 3.22-10.18; p<0.0001), 23.1 mL for basal ganglia ICH (OR 14.11, 95% CI 8.21-24.27; p<0.0001) and 24.4 mL for lobar ICH (OR 8.22, 95% CI 2.3-29.36; p=0.0012). For our secondary outcome, Youden’s lobar ICH cutoff of 42.5mL was the most predictive of mortality. Predictive performances for Youden’s method are shown in Table 1, and for all thresholds shown in Figure 1.
Conclusion
Hematoma volumes associated with poor outcome and mortality vary by location, supporting the notion that different brain regions have different “hematoma volume tolerances”; good outcomes are unlikely when ICH volume exceeds that brain region’s tolerance. Our results provide important data for location-specific hematoma volume tolerance to inform clinical trials and clinical decision rules in ICH management.
More abstracts on this topic:
Nguyen Dan, Ezzeldin Mohamad, Ezzeldin Rime, Mealer Leighann, Mir Osman
A Contemporary Machine Learning-Based Risk Stratification for Mortality and Hospitalization in Heart Failure with Preserved Ejection Fraction Using Multimodal Real-World DataFudim Marat, Weerts Jerremy, Patel Manesh, Balu Suresh, Hintze Bradley, Torres Francisco, Micsinai Balan Mariann, Rigolli Marzia, Kessler Paul, Touzot Maxime, Lund Lars, Van Empel Vanessa, Pradhan Aruna, Butler Javed, Zehnder Tobias, Sauty Benoit, Esposito Christian, Balazard Félix, Mayer Imke, Hallal Mohammad, Loiseau Nicolas
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.