Final ID: TMP46
Comparison of Local and Centrally Adjudicated Modified Rankin Scores in the MOST Trial
Methods: MOST was a Phase 3, single-blind trial evaluating argatroban, eptifibatide, or placebo in addition to standard IV thrombolysis. The primary outcome, the 90-day mRS score, was gathered through in-person video recordings by blinded local personnel. The RFA was encouraged and recordings were sent to central adjudicators for final scoring. During the trial, in-person visits became limited due to the SARS-CoV-2 pandemic; thus, remote telephone or video interviews were allowed. We hypothesized that local mRS scores would be moderately associated with central scores, with no trial outcome differences between local and central mRS. Fleiss-Cohen’s quadratic weighted kappa statistics were used to determine the strength of agreement between local and central scores, as well as by assessment mode, RFA usage, and baseline mRS scores.
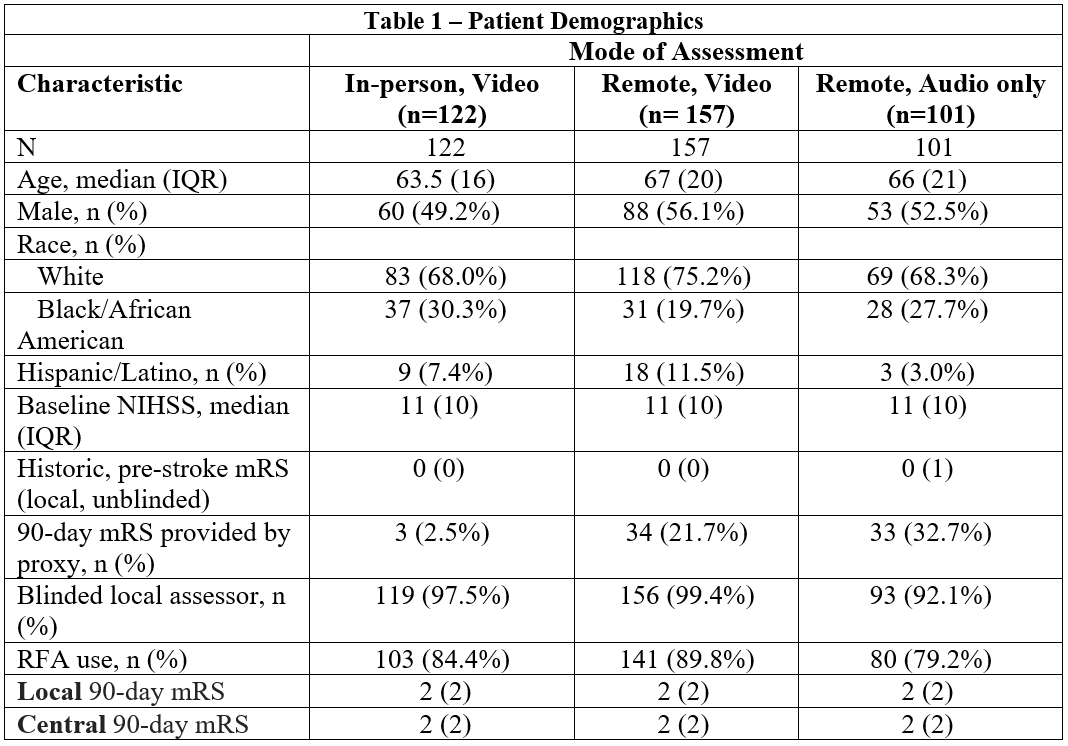
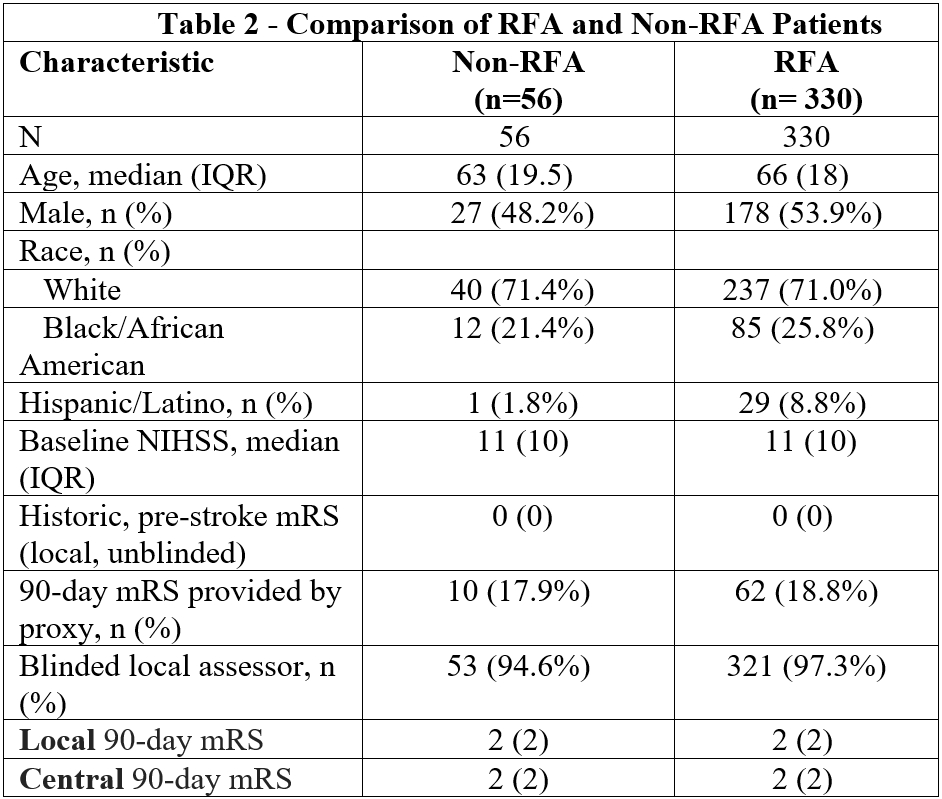
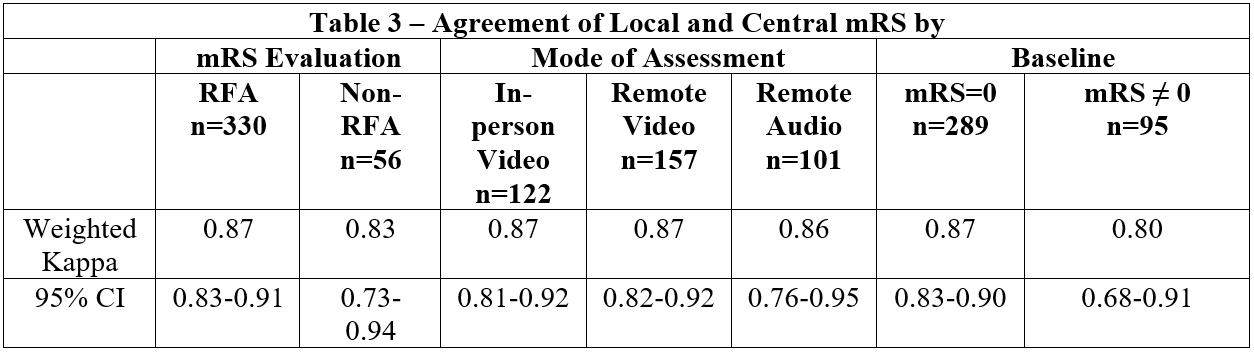
Results: Out of 514 enrolled subjects, 386 subjects had recorded 90-day visits available; 57 died, 8 withdrew consent, 1 terminated early, 26 were lost to follow-up, 33 lacked recorded video/audio, and 3 recordings could not be scored. Of the 386, 122 were in-person video, 157 remote video, and 101 remote audio-only (Table 1). Local assessors were blinded 97.3% of the time and 85.4% of visits used the RFA (Table 2). Overall agreement between local and central mRS scores was very good (weighted Kappa 0.86, 95% CI: 0.82-0.90). However, strength of agreement decreases for those with a baseline mRS greater than zero, (baseline mRS=0: 0.87, 95% CI: 0.83-0.90 versus baseline mRS≠0: 0.80, 95% CI: 0.68-0.91). Agreement by mode of assessment was 0.87 (95% CI: 0.81-0.92) for in-person video, 0.87 (95% CI: 0.82-0.92) for remote video, and 0.86 (95% CI: 0.76-0.95) for remote audio. Trial outcomes were unchanged when utilizing the local mRS versus central adjudication.
Conclusion: Local mRS scores demonstrated strong agreement with central scores across all assessment modes (in-person video, remote video and audio). Caution remains necessary in patients whose baseline mRS is not zero. Central mRS adjudication in acute stroke clinical trials may not be necessary.
More abstracts on this topic:
Alexander Kevin, Bhatt Kunal, Judge Daniel, Grodin Justin, Akinboboye Olakunle, Chen Chris, Tamby Jean-francois, Castano Adam, Fox Jonathan, Fontana Marianna, Gillmore Julian, Sarswat Nitasha, Grogan Martha, Solomon Scott, Davis Margot, Cuddy Sarah, Kittleson Michelle, Shah Keyur, Griffin Jan, Ruberg Frederick, Khouri Michel
A Case Series of Papillary Fibroelastomas on the Coumadin ridgeAboukhatwa Omar, Akiki Elias, Kurmann Reto, Larson Kathryn, Keeney Michael, Bois Melanie, Klarich Kyle
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.