Final ID: TP5
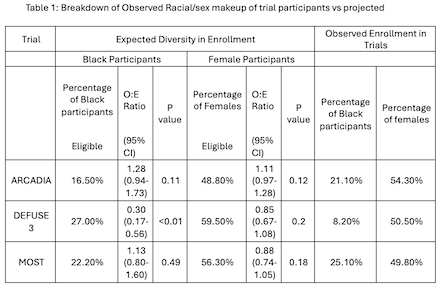
Observed to Expected Sex and Racial Makeup of Trial Participants in Completed StrokeNet Trials
Methods: Since 2014, in collaboration with NIH StrokeNet, we have prepared feasibility analyses for proposed clinical trials. Thus far, 3 trials have been completed: ARCADIA, DEFUSE 3, and MOST. Our analyses used data from the 2010 epoch of the Greater-Cincinnati/Northern Kentucky Stroke Study (GCNKSS). DEFUSE 3 and MOST investigated acute treatments for ischemic stroke while ARCADIA investigated secondary stroke prevention therapies. Each study's inclusion and exclusion criteria were applied to the GCNKSS population and a percentage of eligible patients was generated. Retrospectively, we calculated the proportion of those eligible patients by race and sex and compared that to the observed proportion from each trial using Chi-square test.
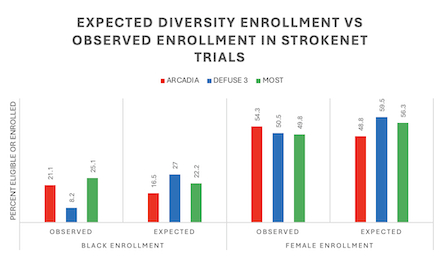
Results: In 2010, there were 2265 ischemic strokes, of which 248, 74, and 135 were predicted to be eligible for ARCADIA, DEFUSE 3, and MOST, respectively. The application of the I/E criteria for each trial was limited by data available in GCNKSS, and all proposed exclusions were not able to be accounted for (namely, imaging data for DEFUSE 3 and EKG/TTE findings for ARCADIA). All 3 trials enrolled an expected number of females but DEFUSE 3 enrolled less Black participants than would have been expected (O:E ratio 0.3, p-value <0.01). Full results are available in Table 1 and Figure 1.
Conclusion: We found no statistically significant difference between the proportion of patients predicted to be eligible stratified by race and sex and the actual proportions of those enrolled for ARCADIA and MOST. For DEFUSE 3, the observed enrollment for black participants was lower than expected. All three trials enrolled near or greater than 50% females. Importantly, site selection may impact a trial’s ability to enroll different minority populations and certain clinical trial exclusion criteria may also asymmetrically affect Black patients and women. These findings should reassure that StrokeNet trials enroll a diverse population.
More abstracts on this topic:
Lane Rashon, Jackson Pasha, Anokwuru Ferdinand, Dillard Naomi, Nerlekar Ridhima
A randomized controlled trial of antithrombotic therapy in ischemic stroke patients with non-valvular atrial fibrillation and atherosclerosis: The ATIS-NVAF trialOkazaki Shuhei, Uchida Kazutaka, Asakura Koko, Omae Katsuhiro, Yamamoto Haruko, Hirano Teruyuki, Toyoda Kazunori, Iguchi Yasuyuki, Noguchi Teruo, Okada Yasushi, Kitagawa Kazuo, Tanaka Kanta, Sakai Nobuyuki, Yamagami Hiroshi, Yazawa Yukako, Doijiri Ryosuke, Koga Masatoshi, Ihara Masafumi, Yamamoto Shiro, Kamiyama Kenji, Honda Yuko
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.