Final ID: 140
Risk of Recurrent Ischemic Stroke Among Patients with Cryptogenic Stroke and Left Ventricular Ejection Fraction
METHODS: We performed a post-hoc exploratory analysis in the ARCADIA trial, a phase III RCT of 1,015 cryptogenic stroke patients with atrial cardiopathy from February 2018 to February 2023. Those with LVEF <30% were not eligible. We dichotomized patients with LVEF into <50% and >50% and built adjusted Cox proportional hazard models to estimate the hazard ratio (HR) of recurrent IS within each LVEF strata by treatment strategy of apixaban versus aspirin. Significance of the interaction was assessed after adjustment for imbalanced covariates found among these groups.
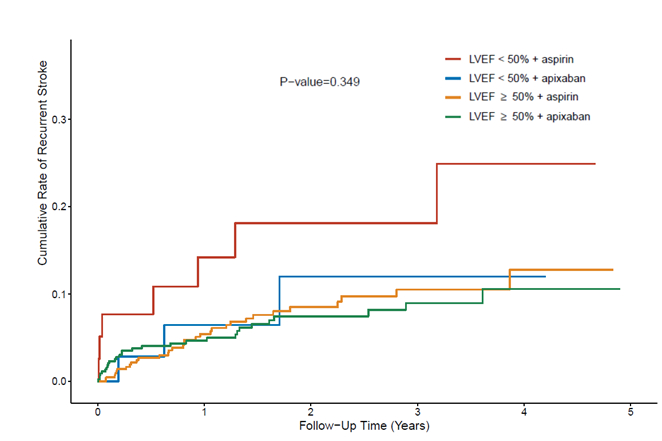
RESULTS: The analytic cohort comprised 963 participants, with LVEF <50% in 74 (7.7%) and ≥50% in 889 (92.3%). Participants with LVEF<50% compared to >50% were younger (65 vs 68 years, p=0.006), more likely male (63.5% vs 44.4%, p=0.002) and of non-White race (45.9% vs 23.8%, p<0.001), and to have coronary artery disease (25.7% vs 8.9%, p<0.001) and CHF (33.8% vs 4.7%, p<0.001), higher N-terminal pro-BNP (median 487 vs 292 pg/mL, p<0.001), larger left atrial diameter index (median 2.1 vs 1.9 cm, p<0.001), and lower LVEF (median 43% vs 62%, p<0.001); P-wave terminal force in V1 was similar. Recurrent IS occurred in 10 (13.5%) patients with LVEF <50% and 61 (6.9%) with LVEF ≥ 50%. The incidence rates of recurrent stroke per 100 person-years were 7.1 (95% CI, 3.8-13.3) with LVEF <50% and 3.9 (95% CI, 3.0-5.0) with LVEF ≥ 50%. In the adjusted analysis, the risk of recurrent stroke was significantly higher with LVEF <50% vs ≥50% (HR 2.23, 95% CI 1.03-4.83). There was no significant interaction between LVEF stratum and the treatment effect (p=0.35; Figure). The risk of recurrent stroke was nominally lower among patients randomized to apixaban than aspirin with LVEF <50% (HR 0.11, 95% C.I. 0.01-1.12), and EF>50% (HR 0.87, 95% C.I. 0.52-1.45) but did not reach statistical significance.
CONCLUSION: Recurrent stroke risk was higher among patients with LVEF <50% vs ≥50%. The risk of recurrent stroke was not different among patients randomized to apixaban compared to aspirin. Further study is needed to identify the optimal anti-thrombotic treatment for patients with cryptogenic stroke and left ventricular dysfunction.
More abstracts on this topic:
Calderon Martinez Ernesto, Camacho Davila Karen Fabiola, Pinto-colmenarez Rafael, Arruarana Victor, Arvelaez Pascucci Joanne, Castillo Jaqueline Livier, Alonso Ramirez Angie Carolina, Ghattas Patricia, Giron De Marza Maria, Sosaya Zuñiga Briggitte Solange, Martinez Lilan Jonathan David, Paredes Romero Enrique
1-Year Outcomes After Cardioversion With and Without Anticoagulation in Patients With Left Atrial Appendage Occlusion: A Propensity-Matched AnalysisThangjui Sittinun, Trongtorsak Angkawipa, Kewcharoen Jakrin, Thyagaturu Harshith, Watson Hangyu, Mensah Samuel, Balla Sudarshan, Navaravong Leenhapong
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.