Final ID: WP51
Non-Traumatic Intracranial Hemorrhage and Risk of Incident Dementia
Methods: We performed a retrospective cohort study using both inpatient and outpatient claims data on Medicare beneficiaries between 2008 and 2018. The exposure was an incident diagnosis of non-traumatic intracranial hemorrhage, defined as a composite of intracerebral hemorrhage, subarachnoid hemorrhage, or subdural hemorrhage. The outcome was an incident diagnosis of dementia. The exposure and outcomes were identified using previously validated ICD-9 and ICD-10-CM diagnosis codes. In the primary analysis, we used Cox regression to study the risk of dementia after any intracranial hemorrhage, after adjusting for demographics and comorbidities. In secondary analyses, the risks of dementia in different subtypes of intracranial hemorrhage were studied.
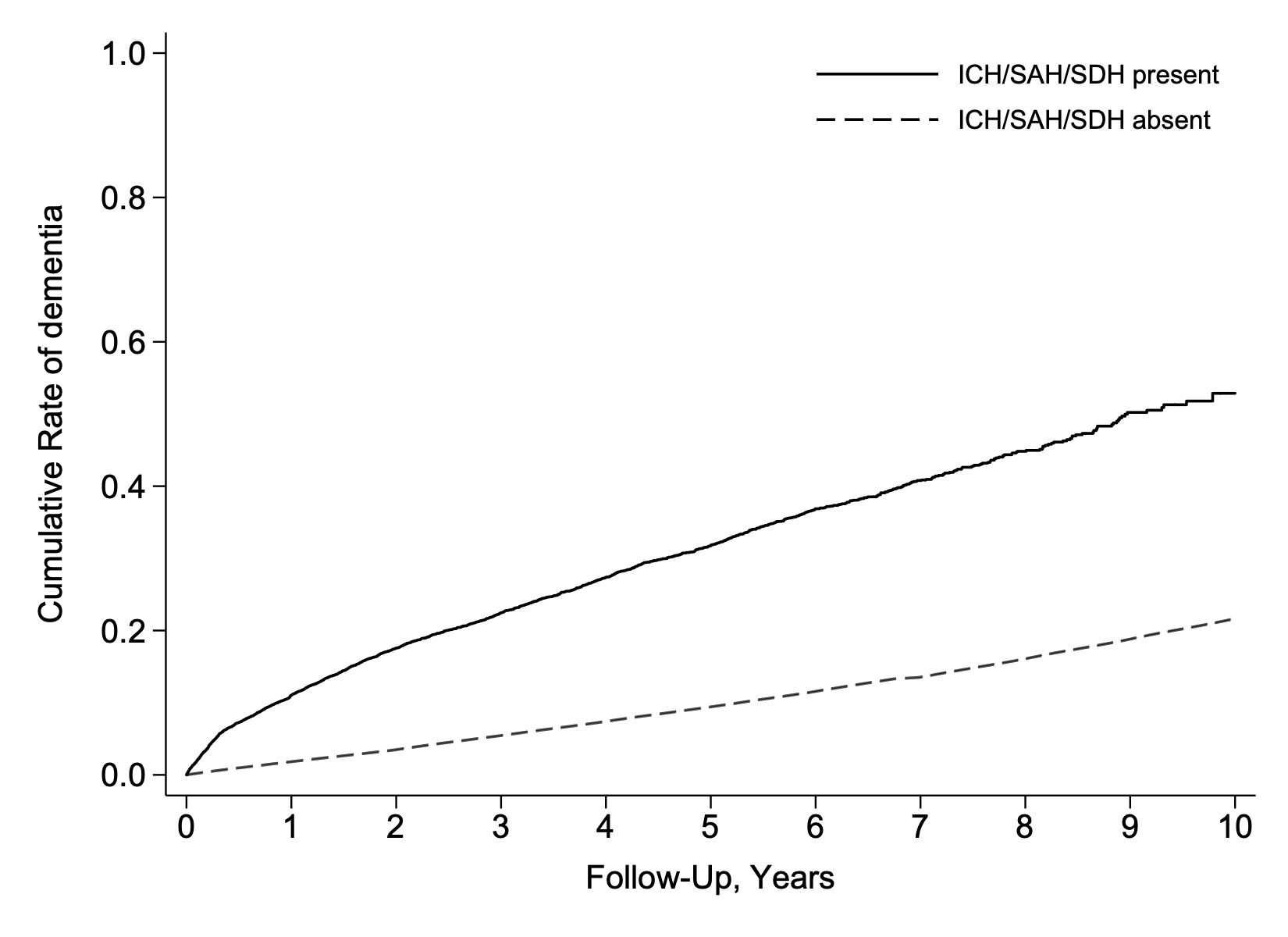
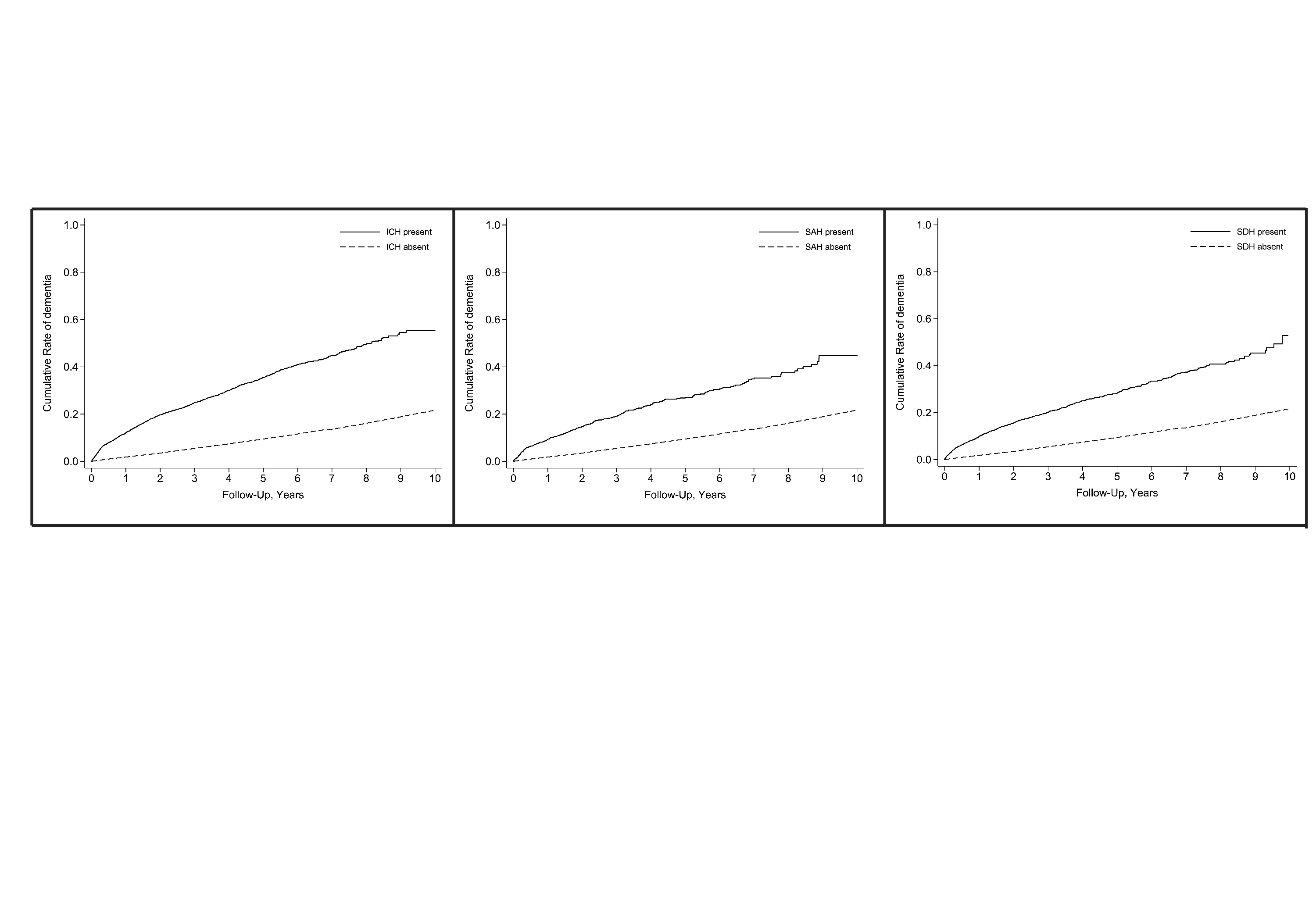
Results: Among 2.1 million patients, 14,775 had a diagnosis of intracranial hemorrhage. During a median follow up of 5.6 years (IQR, 3.0-9.1), incident dementia was diagnosed in 2527 (17.1%) patients with an intracranial hemorrhage and 260,691 (12.8%) patients without. In adjusted Cox regression analysis, intracranial hemorrhage was associated with an increased risk of incident dementia (HR, 2.0; CI, 1.9-2.2). In secondary analyses, a higher risk of incident dementia was observed with intracerebral hemorrhage (HR, 2.4; CI, 2.2-2.5), subarachnoid hemorrhage (HR, 2.0; CI, 1.7-2.2), and subdural hemorrhage (HR, 1.6; CI, 1.4-1.7).
Conclusion: In a large, heterogeneous cohort of elderly US patients, we found that intracranial hemorrhage was independently associated with a 2-fold increased risk of incident dementia. This elevated risk was consistently observed across subtypes of intracranial hemorrhage.
More abstracts on this topic:
Menegat Brenda Luana Rocha Soares, Menegaz De Almeida Artur, Kelly Francinny, A De Moraes Francisco Cezar, Menegat Ana Luiza Rocha Soares, Rocha Dantas Clara, Rachas Reis Mariana, De Souza Wagner Pedro, Simões Bárbara, Alves Mylena, Matheus Gustavo Tadeu, Bezerra Ian
Association Between Circadian Rest/Activity Rhythms and Incident Dementia in Older Adults: The Atherosclerosis Risk in Communities (ARIC) StudyWang Wendy, Crainiceanu Ciprian, Spira Adam, Schrack Jennifer, Chen Lin, Wanigatunga Amal, Etzkorn Lacey, Rabinowitz Jill, Palta Priya, Pike James, Dougherty Ryan, Zipunnikov Vadim, Marino Francesca
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.