Final ID: 044
G6PD-Driven Metabolic Reprogramming Promotes NETosis Formation and Abdominal Aortic Aneurysm Progression
Abstract Body: Introduction Infiltrating neutrophils and neutrophil extracellular traps (NETs) critically exacerbate vascular injury, driving abdominal aortic aneurysm (AAA) initiation and progression. As the pentose phosphate pathway (PPP) rate-limiting enzyme, glucose-6-phosphate dehydrogenase (G6PD) governs neutrophil metabolic plasticity. Excessive activation of PPP mechanistically triggers NETosis. This study investigates G6PD-driven metabolic reprogramming in neutrophils, specifically its causal role in NETs formation and AAA progression.
Hypothesis G6PD drives PPP-dependent NETosis to accelerate AAA.
Methods Serum from healthy volunteers (n=10) and AAA patients (n=10) underwent untargeted and central carbon metabolomics. AAA mouse models: ApoE-/- mice with high-fat diet and AngII pumps; elastase-treated C57BL/6J mice. Mice were intraperitoneally injected with G6PDi-1 (G6PD inhibitor) for 4 weeks. Neutrophils from healthy volunteers were grouped and monitored for cell death and ROS generation using high-content live-cell imaging.
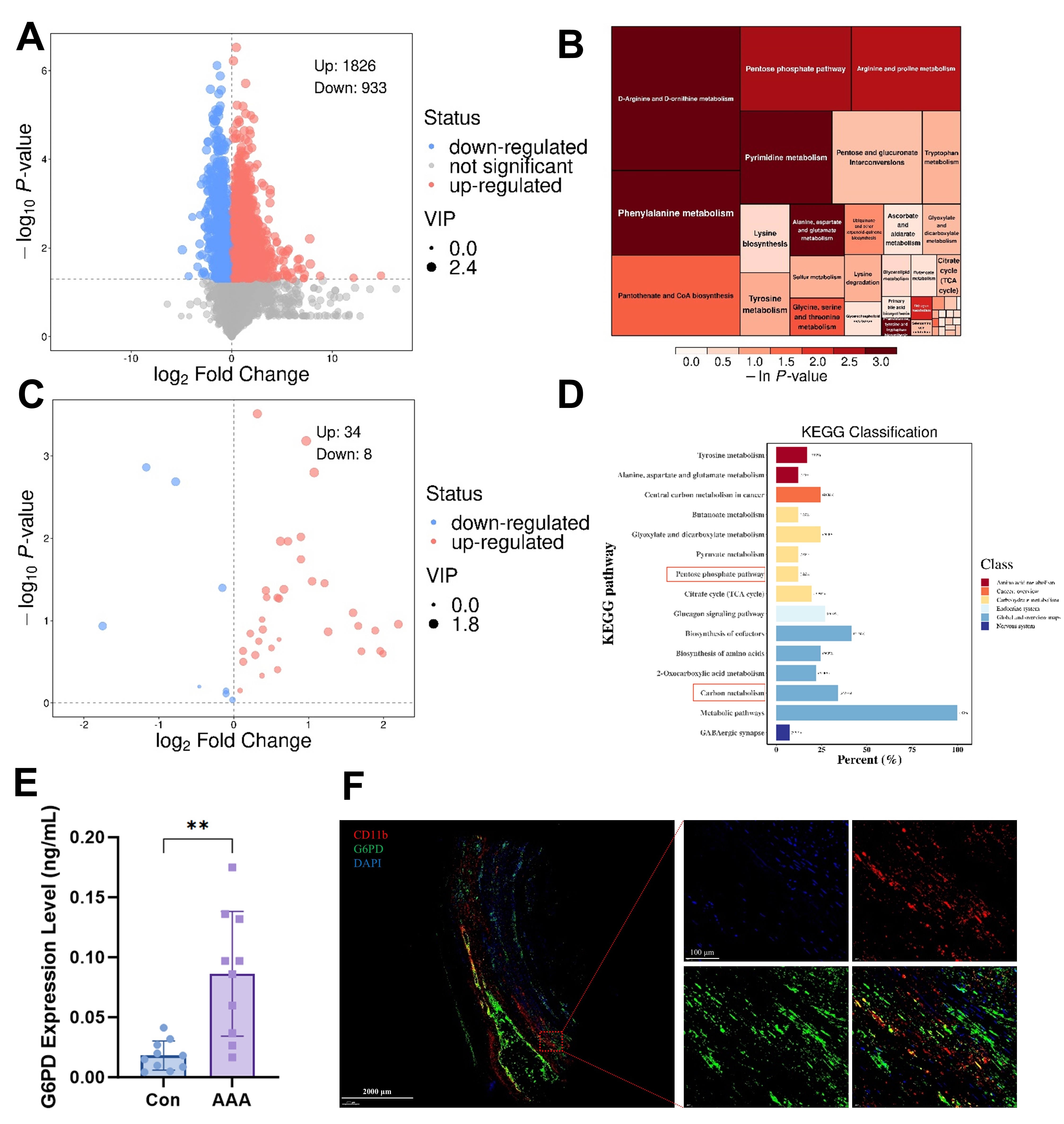
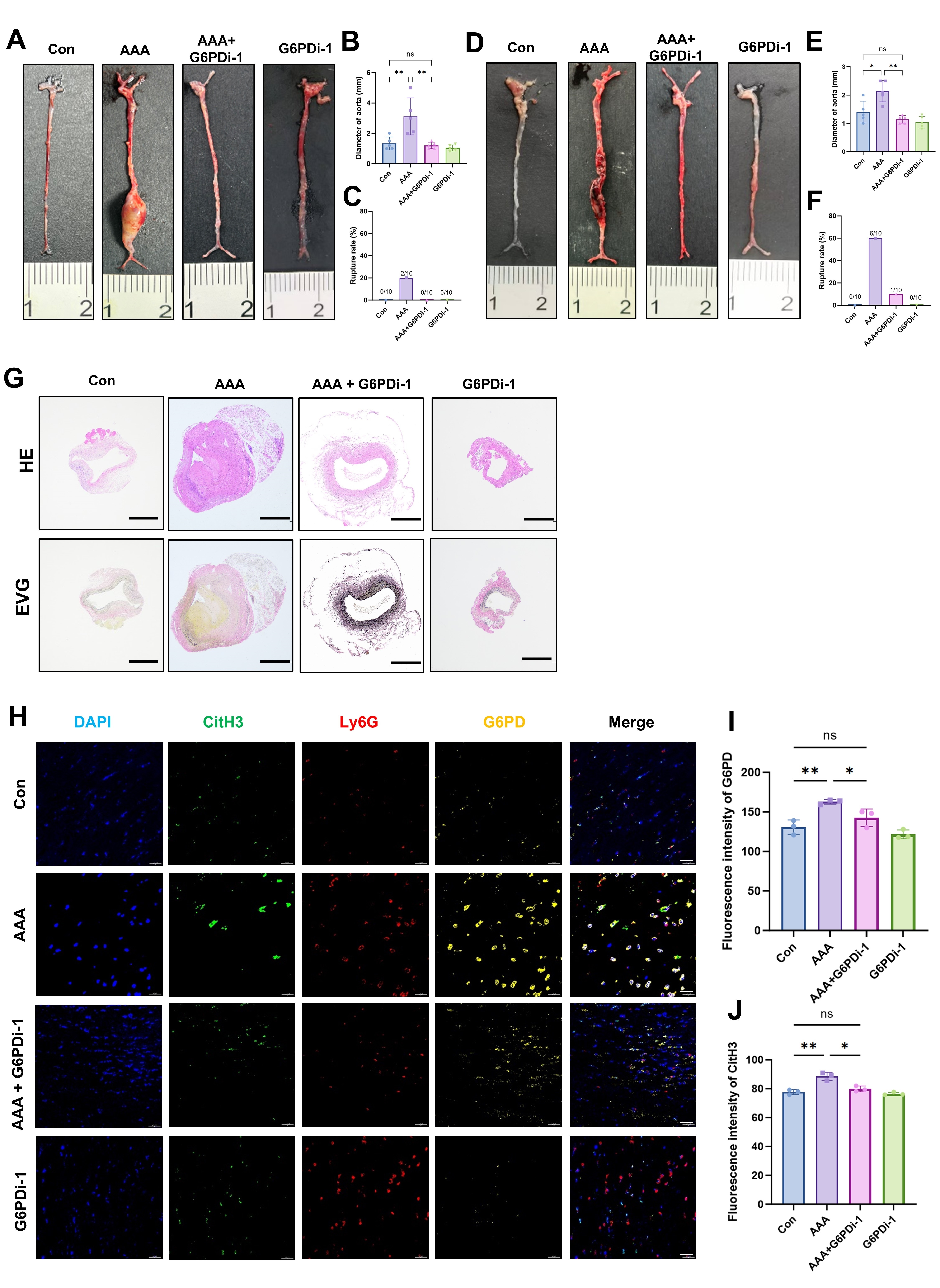
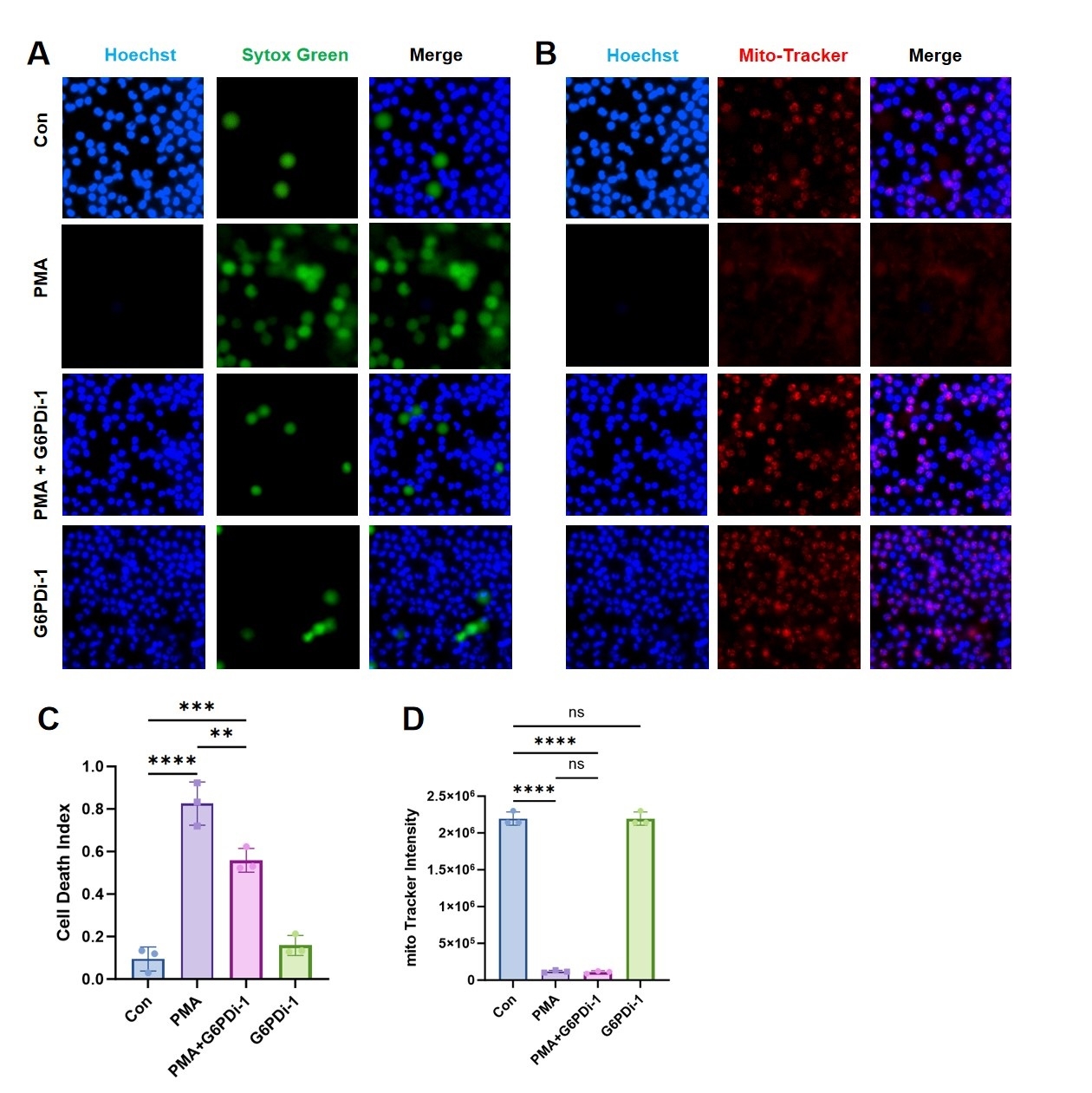
Results Serum PPP activity and G6PD levels were elevated in AAA patients compared to healthy controls (Fig1A–E). G6PD could be co-localized with neutrophils in AAA specimens (Fig1F). Both mouse models developed significant aortic dilation, which was attenuated by G6PDi-1 treatment (Fig2A-F). HE and EVG staining of the ApoE-/- AAA model showed that G6PDi-1 significantly inhibits the fracture and morphological changes of elastic fibers in the vascular wall (Fig2G). The immunofluorescence staining of Ly6G, CitH3, and G6PD showed that G6PDi-1 could inhibit the infiltration of neutrophils in the vascular wall and the NETs formation (Fig2H-J). PMA stimulation triggered NETosis (Fig3A, C), while concomitantly suppressing their ROS-generating capacity (Fig3B, D). Notably, these effects were markedly attenuated by G6PDi-1 co-treatment, demonstrating that pharmacological inhibition of G6PD restores neutrophil viability and ROS production under pro-NETotic conditions.
Conclusion In summary, the PPP pathway of neutrophils is enhanced, and the formation of NETs significantly increases simultaneously during the progression of AAA. Inhibiting G6PD could suppress glucose metabolic reprogramming in neutrophils, thereby attenuating NETosis and ROS production.
Hypothesis G6PD drives PPP-dependent NETosis to accelerate AAA.
Methods Serum from healthy volunteers (n=10) and AAA patients (n=10) underwent untargeted and central carbon metabolomics. AAA mouse models: ApoE-/- mice with high-fat diet and AngII pumps; elastase-treated C57BL/6J mice. Mice were intraperitoneally injected with G6PDi-1 (G6PD inhibitor) for 4 weeks. Neutrophils from healthy volunteers were grouped and monitored for cell death and ROS generation using high-content live-cell imaging.
Results Serum PPP activity and G6PD levels were elevated in AAA patients compared to healthy controls (Fig1A–E). G6PD could be co-localized with neutrophils in AAA specimens (Fig1F). Both mouse models developed significant aortic dilation, which was attenuated by G6PDi-1 treatment (Fig2A-F). HE and EVG staining of the ApoE-/- AAA model showed that G6PDi-1 significantly inhibits the fracture and morphological changes of elastic fibers in the vascular wall (Fig2G). The immunofluorescence staining of Ly6G, CitH3, and G6PD showed that G6PDi-1 could inhibit the infiltration of neutrophils in the vascular wall and the NETs formation (Fig2H-J). PMA stimulation triggered NETosis (Fig3A, C), while concomitantly suppressing their ROS-generating capacity (Fig3B, D). Notably, these effects were markedly attenuated by G6PDi-1 co-treatment, demonstrating that pharmacological inhibition of G6PD restores neutrophil viability and ROS production under pro-NETotic conditions.
Conclusion In summary, the PPP pathway of neutrophils is enhanced, and the formation of NETs significantly increases simultaneously during the progression of AAA. Inhibiting G6PD could suppress glucose metabolic reprogramming in neutrophils, thereby attenuating NETosis and ROS production.
More abstracts on this topic:
Calbindin 2 as A Novel Biomarker and Therapeutic Target for Abdominal Aortic Aneurysm: Integrative Analysis of Human Proteomes and Genetics
Bao Yulin, Zhou Liu-hua, Wang Liansheng
4-Hydroxy-2-Nonenal Alters Alternative Polyadenylation to Regulate mRNA Isoform Diversity in the Transition from Human Cardiac Fibroblasts to MyofibroblastsNatarajan Kartiga, Neupane Rahul, Yalamanchili Hari Krishna, Palaniyandi Suresh, Wagner Eric, Guha Ashrith, Amirthalingam Thandavarayan Rajarajan